Abstract
Phytochemicals are reported to provide various biological functions leading to the promotion of health as well as the reduced risk of chronic diseases. Fat-soluble plant pigments, carotenoids, are extensively studied micronutrient phytochemicals for their potential health benefits. It is noteworthy that specific carotenoids may be responsible for different protective effects against certain diseases. In addition, each carotenoid can be obtained from different types of plant foods. Considering the fact that the phytochemical content in foods can vary according to, but not limited to, the varieties and culture conditions, it is important to establish a database of phytochemicals in locally produced plant foods. Currently, information on individual carotenoid content in plant foods commonly consumed in Korea is lacking. As the first step to support the production and consumption of sustainable local plant foods, carotenoids and total phenolic contents of plant foods commonly consumed in Korea are presented and their potential biological functions are discussed in this review.
Fruits and vegetables containing a wide variety of phytochemicals such as carotenoids and phenolics are consistently reported to reduce the risk of chronic diseases [1-4]. As systemic oxidative stress of cell membranes, DNA, and proteins can contribute to the aging process and risk of various degenerative conditions, the antioxidant functions of phytochemicals may contribute to their protective effect against chronic diseases [5-6]. Indeed, evidence of specific biological functions of various phytochemicals, e.g., anti-inflammation and anti-carcinogenesis, is accumulating [7-14]. In addition, phyrochemicals reported to be involved in direct modulation of signal transduction. In particular, carotenoids such as lycopene [15] and lutein have been suggested to control redox sensitive molecular targets and platelet-derived growth factor [16], respectively.
To understand the biological role of fruits and vegetables and apply this knowledge to human health, it is essential to characterize phytochemicals in plant foods. In an effort to document the bioactive phytochemical contents of foods, a carotenoid database for fruits and vegetables was reported in 1993 based on a review of various publications [17], and updated [18] in 1999 in the US. On the other hand, there is no publication available showing the individual carotenoid content of Korean plant foods except for a booklet written in Korean reported by us [19]. In addition, database for phenolic contents of selected foods have been reported in the US [20-22], France [23-25], and Brazil [26], whereas there is no such data available in Korea. Various plant foods commonly consumed in Korea are not found in Western countries. In addition, phytochemical contents in foods can be significantly different depending on the varieties [27-28], genotypes [29] growing conditions [30] as well as cultivation practices [31]. Therefore the characterization of the major phytochemical contents of fruits and vegetables in Korea is an important step toward understanding the biological functions of plant foods consumed in Korea.
This review provides a spectrum of carotenoids and total phenolics in plant foods commonly consumed in Korea and discusses their potential biological functions.
Phytochemicals have been suggested to provide health benefits such as maintaining inflammation balance [32-33], providing cardiovascular [34-40], neurocognitive [41], and visual health [7-14, 42], and reducing the risk of cancer [43-44]. Even though numerous observational studies suggest that diets high in fruits and vegetables play a role in reducing chronic diseases [14,45-47], several intervention trials failed to show a beneficial effect of relatively high doses of β-carotene (20-30 mg/d) against lung cancer in healthy [48] and high-risk populations [49-50]. Nevertheless, baseline serum β-carotene concentrations are inversely correlated with the subsequent incidence of lung cancer in two of these studies [49-50], suggesting a protective effect of fruit and vegetable consumption on the development of chronic diseases. Thus, it may be due to the combination of various phytochemicals in fruits and vegetables that are required to exert the biological actions that promote health.
For example, antioxidant nutrients in fruits and vegetables can work in a synergistic manner to remove free radicals [51]. Although ascorbic acid is a poor inhibitor of peroxyl radical formation [52], it can effectively recycle α-tocopherol from α-tocopheroxyl radicals (α-TO•) [53]. α-Tocopherol can reduce β-carotene peroxyl radicals (LOO-β-C•) as well as β-carotene radical cations (β-C•+) [54]. The combination of α-tocopherol and β-carotene has been reported to cooperatively slow down lipid peroxidation in in vitro systems [55]. Interestingly, β-carotene at physiologic concentrations does not show a protective effect against oxidation in a biological model system, whereas the oxidation was decreased by β-carotene with the presence of either α-tocopherol or ascorbic acid [51]. Further, flavanol can directly recycle α-tocopherol through a H-transfer mechanism [56]. Importantly, the additive/synergistic interactions between phytochemicals may occur with respect to not only antioxidant activity but also various other biological functions [57].
The various antioxidant actions of carotenoids have been reviewed extensively [58-61], although the existence of a clinical importance of antioxidant effect of these compounds has been questioned by some [62]. Epidemiological studies have suggested that dietary carotenoids play a role in reducing the risk of cancer [63], cardiovascular disease [64-66], macular degeneration [7], and cataracts [8-9]. Specific dietary carotenoids may be responsible for different protective effects. Hydrocarbon carotenoids such as β-carotene may be markers for reduced risk of cancer and heart disease [67-68] in physiological dose, whereas supra dietary doses may lead to increased risk of lung cancer [69-70] as well as gastric cancer [71]. Both epidemiological and laboratory studies consistently indicate an association between oxygenated carotenoids, lutein and zeaxanthin, and the protection of the retina and retinal pigment epithelium from damage induced by UV light and oxygen [10]. For example, Seddon et al. [7] found in a case (n = 356)-control (n = 520) study that the highest quintile of carotenoid intake had a 43% lower risk for age-related macular degeneration compared with those in the lowest quintile, and among the specific carotenoids, lutein and zeaxanthin were most strongly associated with the reduced risk for the disease (P = 0.001). Laboratory human studies identifying the macular pigments as lutein and zeaxanthin support these epidemiologic observations [11-12,72]. However, the intake of dark green vegetables, the major source of lutein, remains low at ~0.2 servings/d in Americans [73]. In addition, data from NHANES III indicate that for women 51-70 y, the medians for 50th & 75th percentiles of lutein/zeaxanthin intake were 1.7 and 2.4 mg/d, respectively [74]. Foods rich in an acyclic carotenoid, lycopene, one of the most abundant carotenoids in human blood and tissues, has been found to be associated with an inverse risk of cervical [75], prostate [76], and colon [77] cancers.
Green leafy vegetables contain both oxygenated and hydrocarbon carotenoids; yellow or orange vegetables have high amounts of β-carotene [78]; tomatoes and watermelon contain high amounts of lycopene [79]. It should be pointed out that most of the measurable carotenoids of human plasma can be increased by moderate alterations in diet within a short time, although the magnitude of the plasma response may be related to the baseline carotenoid concentrations [80].
Phenolic compounds include a vast array of phenolic acids and polyphenols. Total phenolics in fruits and vegetables show a direct relationship with antioxidant capacity measured by in vitro assays [81-82]. The antioxidant and anti-inflammatory activities of plant foods may derive from the additive/synergistic interactions of the mixture of phenolic compounds and other phytochemicals rather than a single comopund or class of compounds [83-84]. Nonetheless, although the data are still limited, some individual phenolic compounds have been reported to show specific biological actions, e.g. anti-inflammation, anti-carcinogenesis.
Flavonoids are a large group of polyphenols present in fruits, vegetables, and beverages including wine and tea as well as tree nuts and whole grains. Flavonols such as quercetin and kaempferol are reported to be rich in onions [21,85] with less amounts in dark green leafy vegetables such as collard greens and kales [86-87]. Various studies have shown an inverse association between quercetin intake and risk of lung cancer [43], cardiovascular diseases [88] and biomarkers of inflammation [13]. Flavanols such as catechins rich in tea and dark chocolate can act to protect unsaturated phospholipids [89] and low-density lipoproteins [90] from oxidation and are correlated with a reduced risk of heart disease [91]. However, controlled intervention trials have been inconsistent in revealing a protective effect of tea drinking against ex vivo LDL oxidation [92-93]. The major food sources for flavanones such as hesperitin and naringenin are citrus fruits and citrus-based juices [94], which are also inversely associated with the risk of cardiovascular disease [95]. Importantly, the daily intake of flavonoids is extremely variable and could range from 25 to 1,000 mg/d [91,96].
WHO reported that up to 2.7 milliion lives could be saved annually with sufficient fruit and vegetable consumption and that low fruit and vegetable intake is among the top 10 selected risk factors for global mortality [97-98]. An WHO/FAO expert consultation report recommends a minimum 400 g (~5 servings) of daily fruits and vegetable intake for the prevention of chronic diseases [99]. As phytochemicals appear to be one of the responsible factors for reducing the risk of chronic diseases, profiling these phytochemicals in Korean fruits and vegetables may help promote consumption of local plant foods as a sustainable option to promote health. Table 1 shows individual carotenoid and total phenolic contents in plant foods commonly consumed in Korea.
The major carotenoids in the fruits and vegetables in plant foods commonly consumed in Korea were found to be β-carotene and lutein. Only 3 and 6 of the studied plant foods contained detectable amounts of trans-lycopene and β-cryptoxanthin, respectively. Fig. 1 shows β-carotene content as the median, 25th and 75th percentiles per 100 g fresh weight (FW) in plant foods grouped by family names. Plant families that contain the highest values of β-carotene were Compositae, Umbelliferae, and Chenopodiaceae in descending order (4.13, 4.00 and 3.10 mg/100 g FW, respectively). Liliaceae, Cruciferae and Solanaceae families contain 1.84, 0.56 and 0.33 mg/100 g FW of β-carotene, respectively. The families containing the lowest β-carotene were Cucurbitaceae, Leguminosae, Rosaceae and Rutaceae families (0.113, 0.064, 0.064 and 0 mg/100 g FW, respectively). The β-carotene content in Umbeliferae family was significantly higher than those in Leguminosae, Rosaceae and Rutaceae families.
Lutein content grouped by plant family is shown in Fig. 2. The Umbelliferae family had the highest lutein content of 7.69 mg/100 g FW, followed by Chenopodiaceae, Compositae and Liliaceae families at 6.27, 5.66 and 2.90 mg/100 g FW, respectively. Cruciferae and Leguminosae families contained 1.0 and 0.80 mg/100 g FW of lutein. Cucurbitaceae, Rosaceae and Rutaceae families contained the lowest amount of lutein in the range of 0-0.14 mg/100 g FW and the content were significantly lower than those in Umbelliferae family.
According to USDA National Nutrient database [100], kale contains 8.17 mg/100 g of β-carotene. The β-carotene contents in pumpkin, carrot, spinach, parsley and lettuce in this database are 6.94, 5.77, 5.63, 5.05 and 4.44 mg/100 g, respectively. Vegetables commonly consumed in Korea are rich in carotenoids, in particular lutein and β-carotene. For example, Myeong Il Yeop and red pepper leaves contained 11.53 and 10.07 mg/100 g of β-carotene and 26.82 and 17.45 mg/100 g of lutein respectively.
Cryptoxanthin and lycopene were detected only in a few food items. Cryptoxanthin was present in red pepper (0.35 mg/100 g), red sweet pepper (0.089 mg/100 g) as well as nectarine, lemon, Japanese apricot and Korean cherry. Lycopene was present in cherry tomato, watermelon and tomato (4.68, 3.33 and 1.94 mg/100 g, respectively).
Umbelliferae (199.2 mg gallic acid equivalents [GAE]/100 g FW), Leguminosae (161.4 mg GAE/100 g FW) and Compositae families (149.3 mg GAE/100 g FW) contained the highest total phenolics, which were significantly higher than Cucurbitaceae family (10.6 mg GAE/100 g FW) containing the lowest total phenolic contents (P < 0.05). Rutaceae, Cruciferae and Chenopodiaceae families were in the range of 65.5-79.7 mg GAE/100 g FW of total phenolics. Solanaceae, Rosaceae, and Liliaceae families contained total phenolics in the amounts of 59.5, 57.5 and 50.7 mg GAE/100 g FW, respectively (Fig. 3).
Previous studies reported by others on total phenolics reported that broccoli and spinach contained the highest amount of total phenolics (101.63 and 90.99 mg GAE/100 g, respectively), followed by yellow onion, red pepper, carrot, cabbage, potato, lettuce, celery and cucumber [101]. Of the 10 most commonly consumed fruits in the US, the highest total phenolic contents were found in cranberry, apple, red grape and strawberry with values of 527.2 ± 21.5, 296.3 ± 6.4, 201.0 ± 2.9, 160.0 ± 1.2 mg GAE/100 g, respectively [102]. Compared to the commonly consumed fruit and vegetables in the US, much higher total phenolics were found in vegetables commonly consumed in Korea, where Gom-chwi (613.79 mg GAE/100 g FW), mugwort (542.81 mg GAE/100 g FW), lactuca bungeama (443.94 mg GAE/100 g FW) and red pepper leaves (409.25 mg GAE/100 g FW), Myeong Il Yeop (331.87 mg GAE/100 g FW), Chwi-namul (274.96 mg GAE/100 g FW ), Dang-gwi (260.53 mg GAE/100 g FW) and small water dropwort (238.65 mg GAE/100 g FW). Korean local berries and legumes which were dried also showed high total phenolics.
The top 20 foods for carotenoids and total phenolics in commonly consumed plant foods in Korea are summarized in Table 2. Myeong Il Yeop and red pepper leaves were the highest in both β-carotene (11.53 and 10.07 mg/100 g FW) and lutein (26.82 and 17.45 mg/100 g FW) contents, followed by perilla leves and mugwort. The top 10 foods containing high amounts of β-carotene were Dang-gwi, Gom-chwi, amanranth, Chwi-namul, parsley and mallow. Pumpkin young leaves, parsley, Dang-gwi, Chwi-namul, amanranth, and Gom-chwi were in top 10 foods with high lutein content.
Total phenolics were the highest in dried Cornus officinalis (Local Korean berry). Gom-chwi (613.8 mg GAE/100 g FW) and mugwort (542.8 µmol/g FW) showed high total phenolics, followed by lactuca bungeama, red pepper leaves, Myeong Il Yeop, Chwi-namul, and Dang-gwi (443.9, 409.3, 331.9, 275 and 260.5 mg GAE/100 g FW, respectively). As shown in Table 2, the top 20 plant foods with high β-carotene content were all green leafy vegetables plus carrots. Lutein was also high in green leafy vegetables. Total phenolics were high in green leafy vegetables and in non-green leafy vegetables such as Korean berry (dried), legumes, grapes, and plum. In general, the phytochemicals investigated in this study were higher in plant foods commonly consumed in Korea, especially in green leafy vegetables, than in common plant foods such as parsley, spinach, kale and lettuce, which are listed to contain high phytochemicals in the US database [21].
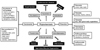
Plant foods commonly consumed in Korea, in particular, green-leafy vegetables belonging to the Compositae and Umbelliferae families, are good sources of phytochemicals such as β-carotene, lutein, and total phenolics, and their contents are higher than those of commonly consumed plant foods reported by others. These phytochemicals in plant foods commonly consumed in Korea may contribute substantially to reduce the risk of chronic diseases as illustrated in Fig. 4. Thus, plant foods commonly consumed in Korea can be an important source of phytochemicals that can contribute to the promotion of health and prevention of chronic diseases.
Figures and Tables
Fig. 1
β-Carotene contents in plant foods commonly consumed in Korea by family names. Umbelliferae (n = 10), Compositae (n = 10), Chenopodiaceae (n = 3), Liliaceae (n = 6), Cruciferae (n = 9), Cucurbitaceae (n = 6), Leguminosae (n = 7), Rosaceae (n = 9), Rutaceae (n = 3), Solanaceae (n = 8). Kruskal-Wallis one-way ANOVA on ranks with Dunn's test were performed to identify differences among median values. Different letters indicate significant differences (P < 0.05).

Fig. 2
Lutein contents in plant foods commonly consumed in Korea by family names. Umbelliferae (n = 10), Compositae (n = 10), Chenopodiaceae (n = 3), Liliaceae (n = 6), Cruciferae (n = 9), Cucurbitaceae (n = 6), Leguminosae (n = 7), Rosaceae (n = 9), Rutaceae (n = 3), Solanaceae (n = 8). Kruskal-Wallis one-way ANOVA on ranks with Dunn's test were performed to identify differences among median values. Different letters indicate significant differences (P < 0.05).
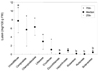
Fig. 3
Total phenolic contents in plant foods commonly consumed in Korea by family names. Umbelliferae (n = 10), Compositae (n = 10), Chenopodiaceae (n = 3), Liliaceae (n = 6), Cruciferae (n = 9), Cucurbitaceae (n = 6), Leguminosae (n = 7), Rosaceae (n = 9), Rutaceae (n = 3), Solanaceae (n = 8). Kruskal-Wallis one-way ANOVA on ranks with Dunn's test were performed to identify differences among median values. Different letters indicate significant differences (P < 0.05).

Notes
This research has been supported in part by Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ008755) Rural Development Administration, Republic of Korea and U.S. Department of Agriculture, under Agreement 58-1950-7-707. The contents of this publication do not necessarily reflect the views or policies of the U.S. Department of Agriculture, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
References
1. Vattem DA, Ghaedian R, Shetty K. Enhancing health benefits of berries through phenolic antioxidant enrichment: focus on cranberry. Asia Pac J Clin Nutr. 2005. 14:120–130.
2. Liu S, Manson JE, Lee IM, Cole SR, Hennekens CH, Willett WC, Buring JE. Fruit and vegetable intake and risk of cardiovascular disease: the Women's Health Study. Am J Clin Nutr. 2000. 72:922–928.

3. Van Duyn MA, Pivonka E. Overview of the health benefits of fruit and vegetable consumption for the dietetics professional: selected literature. J Am Diet Assoc. 2000. 100:1511–1521.

4. Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr. 2003. 78:517S–520S.

6. Willett WC. Balancing life-style and genomics research for disease prevention. Science. 2002. 296:695–698.

7. Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, Farber MD, Gragoudas ES, Haller J, Miller DT, Yannuzzi LA, Willett W. Eye Disease Case-Control Study Group. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. JAMA. 1994. 272:1413–1420.

8. Jacques PF, Chylack LT Jr, Hankinson SE, Khu PM, Rogers G, Friend J, Tung W, Wolfe JK, Padhye N, Willett WC, Taylor A. Long-term nutrient intake and early age-related nuclear lens opacities. Arch Ophthalmol. 2001. 119:1009–1019.

9. Hankinson SE, Stampfer MJ, Seddon JM, Colditz GA, Rosner B, Speizer FE, Willett WC. Nutrient intake and cataract extraction in women: a prospective study. BMJ. 1992. 305:335–339.

10. Hammond BR Jr, Curran-Celentano J, Judd S, Fuld K, Krinsky NI, Wooten BR, Snodderly DM. Sex differences in macular pigment optical density: relation to plasma carotenoid concentrations and dietary patterns. Vision Res. 1996. 36:2001–2012.
11. Bone RA, Landrum JT, Mayne ST, Gomez CM, Tibor SE, Twaroska EE. Macular pigment in donor eyes with and without AMD: a case-control study. Invest Ophthalmol Vis Sci. 2001. 42:235–240.
12. Landrum JT, Bone RA. Lutein, zeaxanthin, and the macular pigment. Arch Biochem Biophys. 2001. 385:28–40.

13. Shaik YB, Castellani ML, Perrella A, Conti F, Salini V, Tete S, Madhappan B, Vecchiet J, De Lutiis MA, Caraffa A, Cerulli G. Role of quercetin (a natural herbal compound) in allergy and inflammation. J Biol Regul Homeost Agents. 2006. 20:47–52.
14. Duthie SJ. Epigenetic modifications and human pathologies: cancer and CVD. Proc Nutr Soc. 2011. 70:47–56.

15. Palozza P, Catalano A, Simone R, Cittadini A. Lycopene as a guardian of redox signalling. Acta Biochim Pol. 2012. 59:21–25.

16. Lo HM, Tsai YJ, Du WY, Tsou CJ, Wu WB. A naturally occurring carotenoid, lutein, reduces PDGF and H(2)O(2) signaling and compromised migration in cultured vascular smooth muscle cells. J Biomed Sci. 2012. 19:18.
17. Mangels AR, Block G, Frey CM, Patterson BH, Taylor PR, Norkus EP, Levander OA. The bioavailability to humans of ascorbic acid from oranges, orange juice and cooked broccoli is similar to that of synthetic ascorbic acid. J Nutr. 1993. 123:1054–1061.
18. Holden JM, Eldridge AL, Beecher GR, Buzzard M, Bhagwat S, Davis CS, Douglass LW, Gebhardt S, Haytowitz D, Schakel S. Carotenoid content of U.S. foods: an update of the database. J Food Compost Anal. 1999. 12:169–196.

19. Cho YS, Park YH, Yoon S, Kim H, Lee-Kim YC, Yeum KJ, Tang G, Blumberg JB, Russell RM. Phytonutrient Contents in Vegetables/Fruits/Legumes. 2009. Seoul: Shinkwang Pub.
20. Nutrient Data Laboratory, Beltsville Human Nutrition Research Center, Agricultural Research Service, U.S. Department of Agriculture. USDA Database for the Proanthocyanidin Content of Selected Foods. 2004. Beltsville: U.S. Department of Agriculture, Agricultural Research Service.
21. Nutrient Data Laboratory, Beltsville Human Nutrition Research Center, Agricultural Research Service, U.S. Department of Agriculture. USDA Database for the Flavonoid Content of Selected Foods - Release 2.1. 2007. Beltsville: U.S. Department of Agriculture, Agricultural Research Service.
22. Nutrient Data Laboratory, Beltsville Human Nutrition Research Center, Agricultural Research Service, U.S. Department of Agriculture. USDA Database for the Isoflavone Content of Selected Foods - Release 2.0. 2008. Beltsville: U.S. Department of Agriculture, Agricultural Research Service.
23. Pérez-Jiménez J, Neveu V, Vos F, Scalbert A. Systematic analysis of the content of 502 polyphenols in 452 foods and beverages: an application of the phenol-explorer database. J Agric Food Chem. 2010. 58:4959–4969.

24. Pérez-Jiménez J, Neveu V, Vos F, Scalbert A. Identification of the 100 richest dietary sources of polyphenols: an application of the Phenol-Explorer database. Eur J Clin Nutr. 2010. 64:Suppl 3. S112–S120.

25. Neveu V, Perez-Jiménez J, Vos F, Crespy V, du Chaffaut L, Mennen L, Knox C, Eisner R, Cruz J, Wishart D, Scalbert A. Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database (Oxford). 2010. 2010:bap024.

26. Arabbi PR, Genovese MI, Lajolo FM. Flavonoids in vegetable foods commonly consumed in Brazil and estimated ingestion by the Brazilian population. J Agric Food Chem. 2004. 52:1124–1131.

27. Correa CR, Li L, Aldini G, Carini M, Chen CY, Chun HK, Cho SM, Park KM, Russell RM, Blumberg JB, Yeum KJ. Composition and stability of phytochemicals in five varieties of black soybeans (Glycine max). Food Chem. 2010. 123:1176–1184.

28. Nisha P, Abdul Nazar P, Jayamurthy P. A comparative study on antioxidant activities of different varieties of Solanum melongena. Food Chem Toxicol. 2009. 47:2640–2644.

29. Lenucci MS, Cadinu D, Taurino M, Piro G, Dalessandro G. Antioxidant composition in cherry and high-pigment tomato cultivars. J Agric Food Chem. 2006. 54:2606–2613.

30. Li L, Aldini G, Carini M, Chen CY, Chun HK, Cho SM, Park KM, Correa CR, Russell RM, Blumberg JB, Yeum KJ. Characterisation, extraction efficiency, stability and antioxidant activity of phytonutrients in Angelica keiskei. Food Chem. 2009. 115:227–232.

31. Wang SY, Chen CT, Sciarappa W, Wang CY, Camp MJ. Fruit quality, antioxidant capacity, and flavonoid content of organically and conventionally grown blueberries. J Agric Food Chem. 2008. 56:5788–5794.

32. González R, Ballester I, López-Posadas R, Suárez MD, Zarzuelo A, Martínez-Augustin O, Sánchez de Medina F. Effects of flavonoids and other polyphenols on inflammation. Crit Rev Food Sci Nutr. 2011. 51:331–362.

33. Basu A, Du M, Sanchez K, Leyva MJ, Betts NM, Blevins S, Wu M, Aston CE, Lyons TJ. Green tea minimally affects biomarkers of inflammation in obese subjects with metabolic syndrome. Nutrition. 2011. 27:206–213.

34. Gresele P, Cerletti C, Guglielmini G, Pignatelli P, de Gaetano G, Violi F. Effects of resveratrol and other wine polyphenols on vascular function: an update. J Nutr Biochem. 2011. 22:201–211.

35. Suri S, Liu XH, Rayment S, Hughes DA, Kroon PA, Needs PW, Taylor MA, Tribolo S, Wilson VG. Quercetin and its major metabolites selectively modulate cyclic GMP-dependent relaxations and associated tolerance in pig isolated coronary artery. Br J Pharmacol. 2010. 159:566–575.

36. Fassett RG, Coombes JS. Astaxanthin: a potential therapeutic agent in cardiovascular disease. Mar Drugs. 2011. 9:447–465.

37. Kuriyama S, Shimazu T, Ohmori K, Kikuchi N, Nakaya N, Nishino Y, Tsubono Y, Tsuji I. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. JAMA. 2006. 296:1255–1265.

38. Knekt P, Kumpulainen J, Järvinen R, Rissanen H, Heliövaara M, Reunanen A, Hakulinen T, Aromaa A. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr. 2002. 76:560–568.

39. Sasazuki S, Kodama H, Yoshimasu K, Liu Y, Washio M, Tanaka K, Tokunaga S, Kono S, Arai H, Doi Y, Kawano T, Nakagaki O, Takada K, Koyanagi S, Hiyamuta K, Nii T, Shirai K, Ideishi M, Arakawa K, Mohri M, Takeshita A. Relation between green tea consumption and the severity of coronary atherosclerosis among Japanese men and women. Ann Epidemiol. 2000. 10:401–408.

40. Hooper L, Kroon PA, Rimm EB, Cohn JS, Harvey I, Le Cornu KA, Ryder JJ, Hall WL, Cassidy A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2008. 88:38–50.

41. Mandel SA, Amit T, Weinreb O, Youdim MB. Understanding the broad-spectrum neuroprotective action profile of green tea polyphenols in aging and neurodegenerative diseases. J Alzheimers Dis. 2011. 25:187–208.

42. Jia Z, Song Z, Zhao Y, Wang X, Liu P. Grape seed proanthocyanidin extract protects human lens epithelial cells from oxidative stress via reducing NF-small ka, CyrillicB and MAPK protein expression. Mol Vis. 2011. 17:210–217.
43. Neuhouser ML. Dietary flavonoids and cancer risk: evidence from human population studies. Nutr Cancer. 2004. 50:1–7.

44. Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004. 74:2157–2184.

45. Li C, Ford ES, Zhao G, Balluz LS, Giles WH, Liu S. Serum alpha-carotene concentrations and risk of death among US Adults: the Third National Health and Nutrition Examination Survey Follow-up Study. Arch Intern Med. 2011. 171:507–515.
46. Liu S, Lee IM, Ajani U, Cole SR, Buring JE, Manson JE. Physicians' Health Study. Intake of vegetables rich in carotenoids and risk of coronary heart disease in men: The Physicians' Health Study. Int J Epidemiol. 2001. 30:130–135.

47. Greenberg ER, Sporn MB. Antioxidant vitamins, cancer, and cardiovascular disease. N Engl J Med. 1996. 334:1189–1190.

48. Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, Belanger C, LaMotte F, Gaziano JM, Ridker PM, Willett W, Peto R. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996. 334:1145–1149.

49. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994. 330:1029–1035.
50. Omenn GS, Goodman G, Thornquist M, Barnhart S, Balmes J, Cherniack M, Cullen M, Glass A, Keogh J, Liu D, Meyskens F Jr, Perloff M, Valanis B, Williams J Jr. Chemoprevention of lung cancer: the beta-Carotene and Retinol Efficacy Trial (CARET) in high-risk smokers and asbestos-exposed workers. IARC Sci Publ. 1996. 67–85.
51. Yeum KJ, Beretta G, Krinsky NI, Russell RM, Aldini G. Synergistic interactions of antioxidant nutrients in a biological model system. Nutrition. 2009. 25:839–846.

52. Doba T, Burton GW, Ingold KU. Antioxidant and co-antioxidant activity of vitamin C. The effect of vitamin C, either alone or in the presence of vitamin E or a water-soluble vitamin E analogue, upon the peroxidation of aqueous multilamellar phospholipid liposomes. Biochim Biophys Acta. 1985. 835:298–303.

53. Liebler DC, Kaysen KL, Burr JA. Peroxyl radical trapping and autoxidation reactions of alpha-tocopherol in lipid bilayers. Chem Res Toxicol. 1991. 4:89–93.

54. Mortensen A, Skibsted LH. Relative stability of carotenoid radical cations and homologue tocopheroxyl radicals. A real time kinetic study of antioxidant hierarchy. FEBS Lett. 1997. 417:261–266.

55. Palozza P, Krinsky NI. beta-Carotene and alpha-tocopherol are synergistic antioxidants. Arch Biochem Biophys. 1992. 297:184–187.
56. Aldini G, Yeum KJ, Carini M, Krinsky NI, Russell RM. (-)-Epigallocatechin-3-gallate prevents oxidative damage in both the aqueous and lipid compartments of human plasma. Biochem Biophys Res Commun. 2003. 302:409–414.

57. Bertipaglia de Santana M, Mandarino MG, Cardoso JR, Dichi I, Dichi JB, Camargo AE, Fabris BA, Rodrigues RJ, Fatel EC, Nixdorf SL, Simão AN, Cecchini R, Barbosa DS. Association between soy and green tea (Camellia sinensis) diminishes hypercholesterolemia and increases total plasma antioxidant potential in dyslipidemic subjects. Nutrition. 2008. 24:562–568.

58. Krinsky NI. The antioxidant and biological properties of the carotenoids. Ann N Y Acad Sci. 1998. 854:443–447.
59. Krinsky NI, Yeum KJ. Carotenoid-radical interactions. Biochem Biophys Res Commun. 2003. 305:754–760.

60. Palozza P, Krinsky NI. Antioxidant effects of carotenoids in vivo and in vitro: an overview. Methods Enzymol. 1992. 213:403–420.
61. Yeum KJ, Aldini G, Russell RM, Krinsky NI. Antioxidant/Pro-oxidant Actions of Carotenoids. 2009. Basel, Boston, Berlin: Birkhauser Verlag.
62. Rice-Evans CA, Sampson J, Bramley PM, Holloway DE. Why do we expect carotenoids to be antioxidants in vivo? Free Radic Res. 1997. 26:381–398.
63. Ziegler RG. Vegetables, fruits, and carotenoids and the risk of cancer. Am J Clin Nutr. 1991. 53:251S–259S.

64. Gaziano JM, Hennekens CH. The role of beta-carotene in the prevention of cardiovascular disease. Ann N Y Acad Sci. 1993. 691:148–155.

65. Riemersma RA, Wood DA, Macintyre CC, Elton RA, Gey KF, Oliver MF. Risk of angina pectoris and plasma concentrations of vitamins A, C, and E and carotene. Lancet. 1991. 337:1–5.

66. Greenberg ER, Baron JA, Karagas MR, Stukel TA, Nierenberg DW, Stevens MM, Mandel JS, Haile RW. Mortality associated with low plasma concentration of beta carotene and the effect of oral supplementation. JAMA. 1996. 275:699–703.

67. Hercberg S, Kesse-Guyot E, Druesne-Pecollo N, Touvier M, Favier A, Latino-Martel P, Briançon S, Galan P. Incidence of cancers, ischemic cardiovascular diseases and mortality during 5-year follow-up after stopping antioxidant vitamins and minerals supplements: a postintervention follow-up in the SU.VI.MAX Study. Int J Cancer. 2010. 127:1875–1881.

68. Kim J, Kim MK, Lee JK, Kim JH, Son SK, Song ES, Lee KB, Lee JP, Lee JM, Yun YM. Intakes of vitamin A, C, and E, and beta-carotene are associated with risk of cervical cancer: a case-control study in Korea. Nutr Cancer. 2010. 62:181–189.

69. Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL Jr, Valanis B, Williams JH Jr, Barnhart S, Cherniack MG, Brodkin CA, Hammar S. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J Natl Cancer Inst. 1996. 88:1550–1559.

70. Albanes D, Heinonen OP, Taylor PR, Virtamo J, Edwards BK, Rautalahti M, Hartman AM, Palmgren J, Freedman LS, Haapakoski J, Barrett MJ, Pietinen P, Malila N, Tala E, Liippo K, Salomaa ER, Tangrea JA, Teppo L, Askin FB, Taskinen E, Erozan Y, Greenwald P, Huttunen JK. Alpha-Tocopherol and beta-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: effects of base-line characteristics and study compliance. J Natl Cancer Inst. 1996. 88:1560–1570.

71. Druesne-Pecollo N, Latino-Martel P, Norat T, Barrandon E, Bertrais S, Galan P, Hercberg S. Beta-carotene supplementation and cancer risk: a systematic review and metaanalysis of randomized controlled trials. Int J Cancer. 2010. 127:172–184.

72. Handelman GJ, Dratz EA, Reay CC, van Kuijk JG. Carotenoids in the human macula and whole retina. Invest Ophthalmol Vis Sci. 1988. 29:850–855.
73. Johnston CS, Taylor CA, Hampl JS. More Americans are eating "5 a day" but intakes of dark green and cruciferous vegetables remain low. J Nutr. 2000. 130:3063–3067.

74. Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. 2000. Washington, D.C.: National Academies Press.
75. Batieha AM, Armenian HK, Norkus EP, Morris JS, Spate VE, Comstock GW. Serum micronutrients and the subsequent risk of cervical cancer in a population-based nested case-control study. Cancer Epidemiol Biomarkers Prev. 1993. 2:335–339.
76. Clinton SK, Emenhiser C, Schwartz SJ, Bostwick DG, Williams AW, Moore BJ, Erdman JW Jr. cis-trans lycopene isomers, carotenoids, and retinol in the human prostate. Cancer Epidemiol Biomarkers Prev. 1996. 5:823–833.
77. Guil-Guerrero JL, Ramos-Bueno R, Rodríguez-García I, López-Sánchez C. Cytotoxicity screening of several tomato extracts. J Med Food. 2011. 14:40–45.

78. Micozzi MS, Beecher GR, Taylor PR, Khachik F. Carotenoid analyses of selected raw and cooked foods associated with a lower risk for cancer. J Natl Cancer Inst. 1990. 82:282–285.

79. Stahl W, Sies H. Uptake of lycopene and its geometrical isomers is greater from heat-processed than from unprocessed tomato juice in humans. J Nutr. 1992. 122:2161–2166.

80. Yeum KJ, Booth SL, Sadowski JA, Liu C, Tang G, Krinsky NI, Russell RM. Human plasma carotenoid response to the ingestion of controlled diets high in fruits and vegetables. Am J Clin Nutr. 1996. 64:594–602.

82. Liu M, Li XQ, Weber C, Lee CY, Brown J, Liu RH. Antioxidant and antiproliferative activities of raspberries. J Agric Food Chem. 2002. 50:2926–2930.

83. de Kok TM, van Breda SG, Manson MM. Mechanisms of combined action of different chemopreventive dietary compounds: a review. Eur J Nutr. 2008. 47:Suppl 2. 51–59.

84. Liu RH. Potential synergy of phytochemicals in cancer prevention: mechanism of action. J Nutr. 2004. 134:3479S–3485S.

85. Somerset SM, Johannot L. Dietary flavonoid sources in Australian adults. Nutr Cancer. 2008. 60:442–449.

86. Lin LZ, Harnly JM. Identification of the phenolic components of collard greens, kale, and Chinese broccoli. J Agric Food Chem. 2009. 57:7401–7408.

87. Olsen H, Aaby K, Borge GI. Characterization and quantification of flavonoids and hydroxycinnamic acids in curly kale (Brassica oleracea L. Convar. acephala Var. sabellica) by HPLC-DAD-ESI-MSn. J Agric Food Chem. 2009. 57:2816–2825.

88. Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993. 342:1007–1011.

89. Chen C, Tang HR, Sutcliffe LH, Belton PS. Green tea polyphenols react with 1,1-diphenyl-2-picrylhydrazyl free radicals in the bilayer of liposomes: direct evidence from electron spin resonance studies. J Agric Food Chem. 2000. 48:5710–5714.

90. Fuhrman B, Aviram M. Flavonoids protect LDL from oxidation and attenuate atherosclerosis. Curr Opin Lipidol. 2001. 12:41–48.

91. Hertog MG, Kromhout D, Aravanis C, Blackburn H, Buzina R, Fidanza F, Giampaoli S, Jansen A, Menotti A, Nedeljkovic S, Pekkarinen M, Simic BS, Toshima H, Feskens EJ, Hollman PC, Katan MB. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch Intern Med. 1995. 155:381–386.
92. Hodgson JM, Puddey IB, Croft KD, Burke V, Mori TA, Caccetta RA, Beilin LJ. Acute effects of ingestion of black and green tea on lipoprotein oxidation. Am J Clin Nutr. 2000. 71:1103–1107.

93. van het Hof KH, de Boer HS, Wiseman SA, Lien N, Westrate JA, Tijburg LB. Consumption of green or black tea does not increase resistance of low-density lipoprotein to oxidation in humans. Am J Clin Nutr. 1997. 66:1125–1132.

94. Zamora-Ros R, Knaze V, Luján-Barroso L, Slimani N, Romieu I, Fedirko V, de Magistris MS, Ericson U, Amiano P, Trichopoulou A, Dilis V, Naska A, Engeset D, Skeie G, Cassidy A, Overvad K, Peeters PH, Huerta JM, Sánchez MJ, Quirós JR, Sacerdote C, Grioni S, Tumino R, Johansson G, Johansson I, Drake I, Crowe FL, Barricarte A, Kaaks R, Teucher B, Bueno-de-Mesquita HB, van Rossum CT, Norat T, Romaguera D, Vergnaud AC, Tjønneland A, Halkjær J, Clavel-Chapelon F, Boutron-Ruault MC, Touillaud M, Salvini S, Khaw KT, Wareham N, Boeing H, Förster J, Riboli E, González CA. Estimated dietary intakes of flavonols, flavanones and flavones in the European Prospective Investigation into Cancer and Nutrition (EPIC) 24 hour dietary recall cohort. Br J Nutr. 2011. 106:1915–1925.

95. Yamada T, Hayasaka S, Shibata Y, Ojima T, Saegusa T, Gotoh T, Ishikawa S, Nakamura Y, Kayaba K. Jichi Medical School Cohort Study Group. Frequency of citrus fruit intake is associated with the incidence of cardiovascular disease: the Jichi Medical School cohort study. J Epidemiol. 2011. 21:169–175.

96. Hollman PC, Feskens EJ, Katan MB. Tea flavonols in cardiovascular disease and cancer epidemiology. Proc Soc Exp Biol Med. 1999. 220:198–202.

97. World Health Organization. The World Health Report 2002 - Reducing Risks, Promoting Healthy Life. 2002. Geneva: World Health Organization.
98. World Health Organization. Fruit and Vegetable Promotion Initiatiative. A Meeting Report. 2003. 2003 Aug 25-27; Geneva: World Health Organization.
99. World Health Organization. Food and Agriculture Organization of the United Nations. Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation. 2003. Geneva: World Health Organization.
100. Haytowitz DB, Lemar LE, Pehrsson PR, Exler J, Patterson KK, Thomas RG, Nickle MS, Williams JR, Showell BA, Khan M, Duvall M, Holden JM. USDA National Nutrient Database for Standard Reference, Release 24. 2011. Beltsville: U.S. Department of Agriculture, Agricultural Research Service.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download