Abstract
This study measured the clinical prevalence of peste des petits ruminants (PPR) among sheep and goats in India between 2003 and 2009 by analyzing clinical samples from suspected cases of PPR that were submitted to the Rinderpest and Allied Disease Laboratory, Division of Virology, IVRI, Mukteswar for PPR diagnosis. PPR outbreaks were confirmed by detecting PPR virus (PPRV)-specific antigen in the clinical samples. Clinical samples (blood, nasal swabs, spleen, lymph node, kidney, liver, intestine, and pooled tissue materials) were taken from a total of 592 sheep and 912 goats in different states of India and screened for the presence of PPRV antigen using a monoclonal antibody-based sandwich ELISA kit. A total of 20, 38, and 11 laboratory-confirmed PPR outbreaks occurred among sheep, goat, and combined sheep and goat populations, respectively. Our findings provide evidence of widespread PPR endemicity in India. The underlying reasons could be variations in husbandry practices in different geographical regions, agro-climatic conditions, and livestock migration. Furthermore, decrease in the number of PPR outbreaks over time might be due to the effectiveness of current live PPR vaccines and timely vaccination of target species. Vaccination against PPR has been practiced in India since 2002 to control this disease.
Peste des petits ruminants (PPR) is an acute, highly contagious disease. The World Organization for Animal Health has identified PPR as a notifiable and economically important transboundary viral disease of sheep and goats associated with high morbidity and mortality [4,16]. Clinically, symptoms of this disease include severe pyrexia, oculo-nasal discharges, necrotizing and erosive stomatitis, enteritis, and pneumonia. The causative agent, PPR virus (PPRV), is genetically grouped into four lineages (I, II, III, and IV) based on partial sequence analysis of the fusion (F) gene. Lineages I to III circulate in Africa while lineage IV is found in Asia [3,9]. However, a recent appearance of lineage IV was associated with a large epizootic in Morocco, and posed a probable risk of introduction to Europe [5]. PPR was first reported in the Ivory Coast of West Africa and was later found in other parts of the world incuding sub-Saharan Africa, the Arabian Peninsula, the Middle East, and the parts of Asia [9,10].
India has a sheep and goat population of approximately 211 million (Ministry of Agriculture, India). Among these livestock, the goat population in India is increasing at a faster rate. Therefore, major emphasis should be given to animal productivity, organized marketing, and prevention of existing and emerging diseases like PPR. In India, PPR was first recorded in the Tamil Nadu state during 1987 and was later an epidemic in northern India. At present, PPR is enzootic in India and outbreaks occur regularly among small ruminants throughout the country [6,11], incurring significant economic losses in terms of morbidity, mortality, and loss of productivity due to trade restriction [11]. Annual losses due to this disease have been estimated at approximately INR 1,800 million (US$ 39 million) with more than 200 million small ruminants at risk [16]. Sequence and phylogenetic analyses of structural protein genes from Indian isolates and vaccine strains of PPRV showed that all viruses have belonged to lineage IV along with other Asian isolates since PPR was first reported [1,3,6,7,9].
Information on prevalence of this disease is available from a number of countries in which the disease has been reported. However, only a systematic study of PPRV infection has been performed in small ruminants from India [11]. Efficient and sensitive diagnostic tests would be very useful for providing quick evidence indicating whether or not PPRV antigens are present in a susceptible population. The clinical prevalence of PPR among sheep and goats could be of epidemiological significance, and data about PPR outbreaks are essential for effective disease management. Therefore, the current study was performed to generate baseline data to determine the prevalence (laboratory confirmed outbreak status) of PPR in India over a period of 6 years.
The PPR outbreaks investigated in this study occurred in various parts of India between 2003 and 2009. Samples were received from numerous organizations such as state disease investigation units and research institutes. Clinical samples such as infected nasal swabs along with spleen, lymph node, and other post-mortem tissues were collected from goat and sheep farms in different geographical locations of the country. These samples were submitted by the Disease Investigation Units of different state Animal Husbandry Departments to the Rinderpest and Allied Disease Laboratory, Division of Virology, Indian Veterinary Research Institute, Mukteswar for analysis and PPR diagnosis. In most cases, the samples used for laboratory confirmation were transported directly via a courrier. Upon receipt, clinical samples were stored at -20℃. Prior to analysis, the tissues samples were tricturated in phosphate buffered saline (PBS) (pH 7.2) and 10% (w/v) suspensions were prepared. The contents of the swab materials were extracted with 500 µL of PBS into eppendorf tubes. All the clinical samples mentioned above were deposited into the virus repository of the Division of Virology, Indian Veterinary Research Institute, Mukteswar (India).
A monoclonal antibody-based sandwich ELISA (s-ELISA) kit for detecting PPRV antigen [12] is commonly used to determine PPR clinical prevalence or diagnose PPR in India. In brief, a capture antibody (anti-rabbit polyclonal antibodies against rinderpest virus) at 1 : 4,000 dilution (100 µL/well) in PBS was used to coat 96-well flat-bottomed ELISA plates (Maxisorp; Nalgene Nunc, Germany). The plates were incubated at 37℃ for 1 h with constant shaking and then washed three times with washing buffer (0.002 mol/L PBS containing 0.05% Tween 20). Suspensions of the clinical samples (50 µL/well) were added to the wells in duplicate. After incubation 37℃ for 1 h and washing three times with washing buffer, a PPRV-specific anti-nucleocapsid protein monoclonal antibody (1 : 20 dilution, 100 µL/well) was added and the plates were incubated 37℃ for 1 h. Anti-mouse horseradish peroxidase conjugates (Sigma-Aldrich, USA) diluted 1 : 1,000 in blocking buffer (PBS containing 0.1% Tween 20 and 0.5% negative serum) were then added (100 µL/well). After incubating at 37℃ for 1 h, substrate solution [0.4 mg/mL o-phenylene diamine (OPD) with 4 µL of 3% H2O2/mL of OPD] was added (100 µL/well) and the plate was incubated at 37℃ for 15 min for color development. The reaction was stopped with 1 M H2SO4 (100 µL/well) before absorbance was read at 492 nm with an ELISA reader (Labsystems Multiskan Plus; Thermo Fisher Scientific, USA).
Estimation of disease prevalence with a 95% confidence interval (CI) and a Chi-square test [13] were performed and data analysis were carried out statistically using statistical analysis system, SAS software (ver. 9.2; SAS, USA).
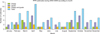
A total of 1,504 clinical samples from sheep (n = 592) and goats (n = 912) in different states of India were screened for PPRV antigen using a PPR-specific s-ELISA kit. Based on our results, the percent of sheep and goats positive for PPR infection were 24.5% [95% CI, 31.86% to 50.16%)] and 38.2% [95% CI, 37.18% to 55.04%], respectively, with an overall percent of 32.8% [95% CI, 36.78% to 50.34%] PPR-positive small ruminants (Table 1). Clinical samples from sheep and goats screened for PPRV antigen and the percent of positive samples are shown in Tables 1~3. There was no significant difference in percent positivity between the various tissue samples screened for PPRV antigen. Additionally, no significant difference in PPRV infection was observed among samples from sheep and goats taken between the years studied or samples from different states of India (Fig. 1). PPR prevalence was high during 2003~2004. This rate was low among sheep during 2006~2007 and goats from 2008~2009. Year refers to the financial period from April to March months. When the temporal distribution of PPR outbreaks was analyzed (Fig. 2), it was found that the number of outbreaks increased during the summer and the September and October months during which wet season occurrs.
For effective control of PPR, accurate diagnostic techniques and timely vaccination of susceptible populations are necessary. Consequently, a full understanding of the disease epidemiology is imperative. PPR eradication depends on rapid and accurate diagnosis, and implementation of prompt control measures. Due to the immense economic impact of PPR, it is absolutely necessary to perform epidemiological surveys of this disease. In India, several PPR outbreaks were not accurately recorded due to inadequate animal disease reporting and surveillance systems. Measuring the clinical prevalence of PPR in different geographical areas of the country with varying agro-climatic conditions may be helpful for establishing disease control strategies and can be useful for determining the actual infection rate. Organized clinical surveys or confirmation of outbreak status has not been performed for PPR in India except for a few isolated clinical reports from different state Animal Husbandry Departments [11]. The majority of these reports except for the one by Singh et al. [11] included only regional data from various states of India since 1994.
The present study has provided preliminary information about confirmed PPR outbreaks or infection among sheep and goats in India between 2003 and 2009. Such information will be very useful for effective disease management and implementing a PPR control program using vaccines against indigenous PPRV. Current vaccination programs are being implemented in some states of India which will alter PPR epidemiology, particularly distribution of the disease. Greater PPR positivity was observed in samples from goats than sheep, which may be due to samples being collected from suspected cases of PPR. Soundararajan et al. [14] also reported a higher mortality rate among infected goats than sheep in a large organised farm that was probably experiencing a PPR outbreak, while analyzing the samples from suspected cases. Based on difference in the virulence of field strains from both species, sheep might have greater innate resistance to clinical development of the disease than goats. This hypothesis appears to be supported by data previously collected during several outbreaks of PPR [11]. It is also possible that the PPRV prefers goat over sheep when both of these natural hosts are reared contiguously. Studies of the molecular aspects of virus affinity and species susceptibility would further elucidate mechanisms underlying the greater PPR susceptibility of goats compared to sheep as described [11].
Besides the regular ante- (blood and swabs) and post-mortem (spleen and lymph nodes) samples, tissues from lung, liver, and heart also showed high positivity for PPRV antigen, showing that the lung, liver, and heart samples from infected animals are also useful for diagnosing PPR. When the tissue samples from sheep and goats were analyzed according to geographic region, high positivity was observed in animals from Tamil Nadu (78.6%) and Uttarakhand (66.2%) compared to other states. Differences in disease prevalence could be due to the sample size. Since relatively small numbers of samples from some states were tested, differences in PPR prevalence among sheep and goats from these various states may not be significant. Low rates of positivity recorded in Punjab and Haryana states in sheep and in Andhra Pradesh in goats. This could be due to small sample sizes, restricted movement of the animals, and decreased transmission of the virus between infected animals. A relatively high proportion (70~80%) of the goat population in India is at risk of infection, particularly in northern parts of the country as reported earlier [11]. Since 1994, a number of PPR outbreaks have been reported in different states of India with variable morbidity and mortality rates [7]. Studies in India and other countries have reported that morbidity and mortality rates vary with different isolates/strains of PPRV in both sheep and goats [1,4,7]. Mortality in susceptible flocks varies from 10 to 100% and morbidity ranges from 50 to 100%. However, this scenario is likely to change drastically once intensive vaccination programs are implemented for the target species. Earlier studies [8,11] indicated that the disease is widely distributed in India, which could probably be attributed to agro-climatic conditions, socio-economic factors, and migration patterns of small ruminants dependent on different factors such as season or flock size.
PPR outbreaks among sheep and goats in India can occur at any time of the year, but are most frequent during the wet (April to September / October) or cold dry (January and February) seasons [11]. When the temporal distribution of PPR outbreaks was analyzed in the current study, increased numbers of outbreaks occurred during the summer and wet seasons. This could be due to limited availability of feed during these periods and close confinement of the animals in farm buildings. However, Taylor [15] believed that increased incidence of the disease might reflect the increased introduction of susceptible young animals to the flocks rather than a seasonal surge in viral activity. This researcher also reported that PPR is mostly observed during the wet season. Moreover, Wosu [17] found that disease incidence peaks during the dry season rather than the rainy season. Inclement dry cold weather during December to February coupled with poor nutrition over this period promotes the spread of PPR.
In India, sheep and goats are reared mostly by nomads. These animals are free-range or pasture-grazed and receive minimum veterinary care, which may further augment the chances of acquiring PPR infection. During the lean period (between March and June), the animals are flocked from one place to another in search of pasture and to be traded. Temporal occurrence of PPR outbreaks correlate with animal movement and climatic factors that favor the survival and spread of the virus. Trading small ruminants at market places, where animals from different locations and sources are brought into close contact with one another, also promotes PPRV transmission [11].
Most investigators have linked PPR outbreaks with the introduction of new animals into flocks. In India, animals are usually under stress during the lean period due to traveling over long distances and nutritional deficiencies. During their migration, these animals frequently infect local populations along the migration route. This may be one of the reasons for the higher frequency of PPR outbreaks among nutritionally deficient animals in which increased susceptibility to infection was previously reported [11]. These infected animals then help to maintain viral circulation throughout the year via frequent animal-to-animal transmission. With commencement of the monsoon season, diets of the animals improve and migration of the animals also ceases due to the availability of local fodder, resulting in substantial decrease in outbreak frequency [11]. Similar observations were also made during a 5-year study of PPR in the tropical humid zone of southern Nigeria [2]. Hence, animal husbandry practices, agro-climatic conditions, and geographical locations impact seasonal distribution of the disease. The movement of animals therefore plays an important role in the transmission and maintenance of PPRV in nature.
In India, present clinical surveillance systems for detecting PPRV antigen by s-ELISA can be synergistically improved with the additional detection of nucleic acid targeting either nucleocapsid or matrix or fusion protein coding gene sequences in clinical samples collected from sheep and goats either suspected of having the disease, are in the incubatory stages, or are at risk of acquiring the disease any time. Perhaps the most effective molecular techniques will be in high-throughput assays such as the identification and monitoring of viruses circulating among free-range sheep and goat populations. Molecular epidemiology will help identify the geographical origin and sources of viruses responsible for the regular outbreaks that occur in endemic countries. In India, decreased number of outbreaks as well as changes in the severity of disease patterns recently observed might be due to the effectiveness of live attenuated vaccines, timely vaccination of sheep and goats, and circulation of a single Asian lineage IV PPRV since the disease was first reported in India [1].
Our findings provide evidence of widespread PPR endemicity in India. The reasons could be variations in husbandry practices in different geographical regions, agro-climatic conditions, and livestock migration. Vaccination against PPR has been practiced in some states of India since 2002 to control the disease [10]. The nation-wide vaccination programme (national control programme of PPR) has been launched during 2010 with an aim to eradicate this disease from India. Currently available PPR vaccines and various PPR-specific diagnostic kits/techniques have been recommended for a collaborative nation-wide control program implemented by state Animal Husbandry Departments under the direction of the Department of Animal Husbandry, Dairying and Fisheries.
Figures and Tables
Table 1
Samples tested to assess the yearly prevalence / outbreaks of peste des petits ruminants (PPR) in sheep and goats*

Table 3
Samples tested for PPR prevalence / outbreaks in sheep and goats according to state*

*Values represent the number (%) of samples positive for PPR infection. **The origin of some of samples submitted by the Centre for Animal Disease Research and Diagnosis, Indian Veterinary Research Institute, Izatnagar in Uttar Pradesh, India was not known, but they were still included in the analysis.
Acknowledgments
The authors thank the Director of the Indian Veterinary Research Institute for providing the facilities necessary for carrying out this work as a service project of the Institute. The authors also thank the staff of Rinderpest and Allied Disease Laboratory, Division of Virology, IVRI (India) for their valuable and timely help with carrying out this study. Finally, the authors thank the Directors of the state Animal Husbandry Departments and Joint Director, Central for Animal Disease Research and Diagnosis (CADRAD), IVRI (India) for sending the samples to the Division of Virology for viral disease diagnosis.
References
1. Balamurugan V, Sen A, Venkatesan G, Yadav V, Bhanot V, Riyesh T, Bhanuprakash V, Singh RK. Sequence and phylogenetic analyses of the structural genes of virulent isolates and vaccine strains of peste des petits ruminants virus from India. Transbound Emerg Dis. 2010. 57:352–364.

2. Butswat ISR, Zahraddeen D, Hussaini AS. Prevalence of peste de pestits ruminant (PPR) and helminthiasis in sheep and goats in Bauchi, Nigeria. Bull Anim Health Prod Afr. 2005. 53:131–134.
3. Dhar P, Sreenivasa BP, Barrett T, Corteyn M, Singh RP, Bandyopadhyay SK. Recent epidemiology of peste des petits ruminants virus (PPRV). Vet Microbiol. 2002. 88:153–159.

4. Diallo A, Minet C, Le Goff C, Berhe G, Albina E, Libeau G, Barrett T. The threat of peste des petits ruminants: progress in vaccine development for disease control. Vaccine. 2007. 25:5591–5597.

5. FAO Animal Production and Health Division. Peste des petits ruminants (PPR): an incresing threat to small ruminant production in Africa and Asia. Transbound Anim Dis Bull. 2009. 33:2–8.
6. Kerur N, Jhala MK, Joshi CG. Genetic characterization of Indian peste des petits ruminants virus (PPRV) by sequencing and phylogenetic analysis of fusion protein and nucleoprotein gene segments. Res Vet Sci. 2008. 85:176–183.

7. Nanda YP, Chatterjee A, Purohit AK, Diallo A, Innui K, Sharma RN, Libeau G, Thevasagayam JA, Brüning A, Kitching RP, Anderson J, Barrett T, Taylor WP. The isolation of peste des petits ruminants virus from Northern India. Vet Microbiol. 1996. 51:207–216.

8. Raghavendra AG, Gajendragad MR, Sengupta PP, Patil SS, Tiwari CB, Balumahendiran M, Sankri V, Prabhudas K. Seroepidemiology of peste des petits ruminants in sheep and goats of southern peninsular India. Rev Sci Tech. 2008. 27:861–867.

9. Shaila MS, Shamaki D, Forsyth MA, Diallo A, Goatley L, Kitching RP, Barrett T. Geographic distribution and epidemiology of peste des petits ruminants viruses. Virus Res. 1996. 43:149–153.

10. Singh RK, Balamurugan V, Bhanuprakash V, Sen A, Saravanan P, Yadav MP. Possible control and eradication of peste des petits ruminants from India: technical aspects. Vet Ital. 2009. 45:449–462.
11. Singh RP, Saravanan P, Sreenivasa BP, Singh RK, Bandyopadhyay SK. Prevalence and distribution of peste des petits ruminants virus infection in small ruminants in India. Rev Sci Tech. 2004. 23:807–819.

12. Singh RP, Sreenivasa BP, Dhar P, Bandyopadhyay SK. A sandwich-ELISA for the diagnosis of Peste des petits ruminants (PPR) infection in small ruminants using anti-nucleocapsid protein monoclonal antibody. Arch Virol. 2004. 149:2155–2170.

13. Snedecor GW, Cochran WG, editors. Statistical Methods. 1967. 6th ed. Ames: Iowa State Univ. Press;20–31.
14. Soundararajan C, Sivakumar T, Ramesh S, Muthukrishnan S, Palanidorai R. Peste des petits ruminants among sheep and goats in an organized farm in Tamil Nadu. Indian Vet J. 2006. 83:1045–1047.
15. Taylor WP. The distribution and epidemiology of peste des petits ruminants. Prev Vet Med. 1984. 2:157–166.

16. Venkataramanan R, Bandyopadhyay SK, Oberoi MS. Present status and strategies for the control of transboundary and other economically important animal diseases in India: a review. Indian J Anim Sci. 2005. 75:456–464.
17. Wosu LO. Current status of peste des petits ruminants (PPR) disease in small ruminants: a review article. Stud Res Vet Med. 1994. 2:83–90.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download