Abstract
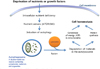
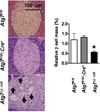
Diabetes mellitus is characterized by decreased insulin secretion and action. Decreased insulin secretion results from a reduction in mass and/or function of pancreatic β-cells. Apoptosis, oxidative stress, mitochondrial dysfunction, and endoplasmic reticulum (ER) stress responses have been suggested as mechanisms for the changes in β-cells in type 2 diabetes; however, the underlying causes have not been clearly elucidated. Autophagy is an intracellular process that maintains cellular homeostasis through degradation and recycling of organelles. Recently, we reported reduction of β-cell mass in autophagy-deficient mice. Pancreatic insulin content was also decreased due to the decreased β-cell mass and the reduced number of insulin granules. Morphological analysis of these β-cells revealed an accumulation of ubiquitinated proteins, swollen mitochondria, and distended ER. Insulin secretory function ex vivo was also impaired. As a result, autophagy-deficient mice showed hypoinsulinemia and hyperglycemia. These results suggested that autophagy is necessary to maintain the structure, mass and function of β-cells. In addition, as autophagy may play a protective role against ER stress and rejuvenate organelle function, impaired autophagy may lead to mitochondrial dysfunction and ER stress, which have been implicated as causes of insulin resistance. Therefore, in addition to β-cell homeostasis, dysregulated autophagy may possibly be involved in insulin resistance.
Figures and Tables
References
1. Boya P, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, Metivier D, Meley D, Souquere S, Yoshimori T, Pierron G, Codogno P, Kroemer G. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005. 25:1025–1040.
2. Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, Thompson CB. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005. 120:237–248.
3. Ashford TP, Porter KR. Cytoplasmic components in hepatic cell lysosomes. J Cell Biol. 1962. 12:198–202.
4. Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008. 132:27–42.
5. Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest. 2005. 115:2679–2688.
6. Kaniuk NA, Kiraly M, Bates H, Vranic M, Volchuk A, Brumell JH. Ubiquitinated-protein aggregates form in pancreatic beta-cells during diabetes-induced oxidative stress and are regulated by autophagy. Diabetes. 2007. 56:930–939.
7. Jung HS, Chung KW, Kim JW, Kim J, Komatsu M, Tanaka K, Nguyen YH, Kang TM, Yoon KH, Kim JW, Jeong YT, Han MS, Lee MK, Kim KW, Shin J, Lee MS. Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell Metab. 2008. 8:318–324.
8. Ebato C, Uchida T, Arakawa M, Komatsu M, Ueno T, Komiya K, Azuma K, Hirose T, Tanaka K, Kominami E, Kawamori R, Fujitani Y, Watada H. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metab. 2008. 8:325–332.
9. Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006. 441:885–889.
10. Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T, Mizushima N, Iwata J, Ezaki J, Murata S, Hamazaki J, Nishito Y, Iemura S, Natsume T, Yanagawa T, Uwayama J, Warabi E, Yoshida H, Ishii T, Kobayashi A, Yamamoto M, Yue Z, Uchiyama Y, Kominami E, Tanaka K. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell. 2007. 131:1149–1163.
11. Bernales S, Schuck S, Walter P. ER-phagy: selective autophagy of the endoplasmic reticulum. Autophagy. 2007. 3:285–287.
12. Rodriguez-Enriquez S, Kim I, Currin RT, Lemasters J. Tracker dyes to probe mitochondrial autophagy (mitophagy) in rat hepatocytes. Autophagy. 2006. 2:39–46.
13. Masini M, Bugliani M, Lupi R, del Guerra S, Boggi U, Filipponi F, Marselli L, Masiello P, Marchetti P. Autophagy in human type 2 diabetes pancreatic beta cells. Diabetologia. 2009. 52:1083–1086.
14. Han D, Yang B, Olson LK, Greenstein A, Baek SH, Claycombe KJ, Goudreau JL, Yu SW, Kim EK. Activation of autophagy through modulation of AMP-activated protein kinase protects pancreatic beta-cells from hyperglycemia. Biochem J. 2009. 11. 11. [Epub ahead of print].
15. Choi SE, Lee SM, Lee YJ, Li LJ, Lee SJ, Lee JH, Kim Y, Jun HS, Lee KW, Kang Y. Protective role of autophagy in palmitate-induced INS-1 beta-cell death. Endocrinology. 2009. 150:126–134.
16. Fujimoto K, Hanson PT, Tran H, Ford EL, Han Z, Johnson JD, Schmidt RE, Green KG, Wice BM, Polonsky KS. Autophagy regulates pancreatic beta cell death in response to Pdx1 deficiency and nutrient deprivation. J Biol Chem. 2009. 284:27664–27673.
17. Salminen A, Kaarniranta K. Regulation of the aging process by autophagy. Trends Mol Med. 2009. 15:217–224.
18. Rajawat YS, Hilioti Z, Bossis I. Aging: central role for autophagy and the lysosomal degradative system. Ageing Res Rev. 2009. 8:199–213.
19. Zhou L, Zhang J, Fang Q, Liu M, Liu X, Jia W, Dong LQ, Liu F. Autophagy-mediated insulin receptor down-regulation contributes to endoplasmic reticulum stress-induced insulin resistance. Mol Pharmacol. 2009. 76:596–603.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


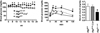
 XML Download
XML Download