Abstract
After mastectomy and axillary node dissection, chylous leakage is rare. However, considerable anatomical variation in the termination of the thoracic duct has been reported. Hence, during breast surgery, injury to the lateral terminating branch is not unlikely and might lead to retrograde chyle leak. Herein, we describe a patient who had a chylous leakage at her wound site after a left simple mastectomy and axillary node dissection and for whom lymphoscintigraphy with Tc-99m albumin nanocolloid was performed. In this case, additional hybrid single-photon emission computed tomography/computed tomography study was done, and has helped with the accurate identification of the chyle leakage site, thus aiding in surgical management.
Chyle leakage has been known to result from damage to the thoracic duct or its branches during neck or thoracic surgery [1]. However, postmastectomy chyle leakage with axillary clearance is a rare phenomenon; chronic lymphoedema or seroma formation in the upper limb is a more commonly seen complication. Lymphoscintigraphy has been found to be useful for the detection of such chylous leaks [2]. Herein, we describe the novel use of additional hybrid single-photon emission computed tomography/computed tomography (SPECT/CT) imaging during lymphoscintigraphy to further evaluate and localize a postmastectomy chyle leakage with axillary clearance. This technique helped in the surgical management and resulted in a successful outcome for our patient.
A 78-year-old woman with ductal carcinoma of the left breast underwent a simple mastectomy and axillary node dissection. Five days following surgery, she noticed an increasing fluctuant lump at the mastectomy site. The aspirated fluid had a "milky" appearance and was found to be suggestive of a thoracic duct leak. The patient underwent lymphoscintigraphy with 74 megabecquerels of Tc-99m albumin nanocolloid that was administered subdermally in the second webspace of the left hand. The particle size of the Tc-99m albumin nanocolloid used in this case (more than 80% of them) was less than 100 nanometers. The initial dynamic images showed a prompt migration of tracer along the lymphatic trunks of the left upper limb, leading to a small focal collection in the left axilla within 30 minutes (Figure 1). At 90 minutes, no other suspicious tracer uptake was seen. A second tracer injection was attempted in the left foot to increase the tracer flow in the thoracic duct and the likelihood of visualization [3].
Satisfactory cephalic migration of the tracer was seen in the left lower limb, up to the inguinal nodes. At 3 hours, mild diffuse tracer uptake was noted at the left breast, compatible with a chyle leak (Figure 2). Another tiny focal tracer uptake was seen inferiorly. There was mild visualization of the liver. No significant lymphatic tract was seen in the abdomen at this point.
At 4 hours, delayed images were obtained and showed increasing tracer intensity at the left axilla and left breast (Figure 3). SPECT/CT examination was performed to further assess the focal uptake located superior and inferior to the chyle collection.
SPECT/CT showed a large fluid collection (18×6 cm) with mild tracer uptake at the mastectomy site that was consistent with a chyle leak. The tracer uptake inferior to the chyle collection on planar imaging was found to be located within the collection itself on SPECT/CT, likely representing nonuniformity of chyle concentration (Figure 4). Focal uptake at the left axilla did not correlate with any obvious anatomical structures, but SPECT/CT localized this focus to be beyond the left subclavian vein, anterior to the left axillary vein, and inferior to the left biceps tendon, likely representing a leakage from a tiny branch of the thoracic duct. The tracer uptake gradually emptied into the chyle collection.
The patient underwent re-exploration of the wound, and 800 mL of chyle was drained. A small recurrent collection that was suspected to be a chyle leak was immediately seen within a 5×3-cm cavity inferior to the biceps tendon, as confirmed by SPECT/CT scan. Tissue glue was applied to the cavity, followed by surgical closure of and a compression dressing to the wound. The patient progressed well postoperatively with no additional immediate complications.
A postmastectomy chyle leakage with axillary clearance is rare because of the anatomical distance between the thoracic duct and the operation site, with a reported incidence of <0.5% [4,5,6]. Considerable anatomical variation in the termination of the thoracic duct has been reported [1,7]. The lateral terminating branch is susceptible to injury during breast surgery, leading to retrograde chyle leak. The presence of isotope activity in the liver further suggested that the injury was likely to be of a laterally terminating branch, preserving some communication between the thoracic duct flow and the venous system. Initially, a chylous leakage can be managed conservatively [5], although some authors advocate timely surgical management because of its low associated morbidity and to prevent delays in further oncological treatment [8].
To the best of our knowledge, there has been only one published article in which lymphoscintigraphy was used to diagnose chyle leak after breast surgery [2]. However, no report has been published on the utility of SPECT/CT in this context. Hybrid SPECT/CT imaging has seen incremental growth in the past decade following the advancement and widespread utilization of positron emission tomography/computed tomography [9]. SPECT imaging improves localization, as compared with planar scintigraphy, by allowing a three-dimensional view of the lesion of interest. However, SPECT does not provide precise anatomical information. In the late 1990s, SPECT and CT images were brought together and fused to overcome the lack of anatomical landmarks. By accurately characterizing areas of abnormal tracer uptake, SPECT/CT improves specificity and sensitivity and thus tremendously improves diagnostic accuracy, as compared with planar study or SPECT alone [10,11].
Figures and Tables
Figure 1
Initial dynamic images of Tc-99m albumin nanocolloid planar lymphoscintigraphy obtained after tracer injection in the left hand. The images showed prompt migration of the tracer along the lymphatic trunks of the left upper limb, leading to a small focal collection in the left axilla within 30 minutes.

Figure 2
Tc-99m albumin nanocolloid planar lymphoscintigraphy obtained at 3 hours, after a second tracer injection in the left foot. (A) Mild diffuse tracer uptake at the left breast is noted (hollow arrow), compatible with a chyle leak. Another tiny focal tracer uptake (solid arrow) was seen inferiorly. (B) Image with background flood source.
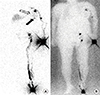
Figure 3
Tc-99m albumin nanocolloid planar lymphoscintigraphy obtained at 4 hours. (A) Delayed images obtained at 4 hours showed increasing tracer intensity at the left axilla (thin arrow) and left breast (thick arrow). (B) Image with background flood source.

Figure 4
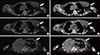
Single-photon emission computed tomography/computed tomography (SPECT/CT) scan of the thorax. (A) SPECT/CT reveals focal uptake at the left axilla (arrow). (B) Correlative CT scan localized this uptake to be beyond the left subclavian vein, anterior to the left axillary vein, and inferior to the left biceps tendon (arrow). (C, D) This tracer uptake empties into the chyle collection (arrows). (E, F) Tracer uptake inferior to the chyle collection locates within the collection itself (arrow), as confirmed by correlative CT scan (arrow), likely representing nonuniformity of the chyle concentration.
References
2. Abdelrazeq AS. Lymphoscintigraphic demonstration of chylous leak after axillary lymph node dissection. Clin Nucl Med. 2005; 30:299–301.

3. Pui MH, Yueh TC. Lymphoscintigraphy in chyluria, chyloperitoneum and chylothorax. J Nucl Med. 1998; 39:1292–1296.
4. Fitz-Hugh GS, Cowgill R. Chylous fistula. Arch Otolaryngol. 1970; 91:543–547.
5. Nakajima E, Iwata H, Iwase T, Murai H, Mizutani M, Miura S, et al. Four cases of chylous fistula after breast cancer resection. Breast Cancer Res Treat. 2004; 83:11–14.

6. Singh M, Deo SV, Shukla NK, Pandit A. Chylous fistula after axillary lymph node dissection: incidence, management, and possible cause. Clin Breast Cancer. 2011; 11:320–324.

7. Rice DC, Emory RE Jr, McIlrath DC, Meland NB. Chylous fistula: an unusual occurrence after mastectomy with immediate breast reconstruction. Plast Reconstr Surg. 1994; 93:399–401.
8. Baek JM, Lee JA, Nam YH, Sung GY, Lee do S, Won JM. Chylous leakage: a rare complication after axillary lymph node dissection in breast cancer and surgical management. J Breast Cancer. 2012; 15:133–134.

9. Mariani G, Bruselli L, Kuwert T, Kim EE, Flotats A, Israel O, et al. A review on the clinical uses of SPECT/CT. Eur J Nucl Med Mol Imaging. 2010; 37:1959–1985.

10. Shreve PD. Adding structure to function. J Nucl Med. 2000; 41:1380–1382.
11. Schillaci O, Simonetti G. Fusion imaging in nuclear medicine: applications of dual-modality systems in oncology. Cancer Biother Radiopharm. 2004; 19:1–10.

12. Ohtsuka A, Inoue Y, Asano Y, Woodhams R, Shiomi K. Lymphoscintigraphy using dynamic imaging and SPECT/CT in chylothorax. Open J Med Imaging. 2013; 3:86–89.

13. Kotani K, Kawabe J, Higashiyama S, Shiomi S. Lymphoscintigraphy with single-photon emission computed tomography/computed tomography is useful for determining the site of chyle leakage after esophagectomy. Indian J Nucl Med. 2012; 27:208–209.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download