Abstract
The perivascular spaces (PVSs) of the brain are lined with pia and contain interstitial fluid. In general, PVSs are small, asymptomatic, and identifiable at all ages. When PVSs are significantly enlarged, they can produce various clinical manifestations such as headache and dizziness. A 67-year-old man was admitted with cognitive impairment and gait disturbance with a 5-month history. Brain MRI showed multiple cystic PVSs in periventricular and subcortical white matter of both hemispheres. Medication with dopaminergic agents produced a moderate clinical improvement, while anticholinesterase was not effective. This case suggests that disseminated polycystic dilated PVSs may present with dementia and Parkinsonism.
The perivascular spaces (PVSs) of the brain, also known as Virchow-Robin spaces, are lined with pia and contain interstitial fluid (ISF).1 These structures can be identified in normal brains of various ages using brain magnetic resonance imaging (MRI). Most PVSs are small and asymptomatic, and are considered as benign structures. However, if they are dilated, various manifestations including headache, dizziness, and memory impairment may develop.1,2 Rarely, PVSs may present with widespread multiple cysts in bizarre configurations, and thus be mistaken as more ominous diseases such as cystic neoplasm.3 We report a patient with disseminated polycystic dilated PVSs presenting with progressive dementia and parkinsonian features.
A 67-year-old man was admitted to our hospital with progressive memory impairment and gait disturbance with a 5-month history. The patient had no surgical or medical history, he had never taken antidopaminergic drugs or drugs affecting cognition, and his family history was unremarkable. A neurological examination showed a masked face, bilateral rigidity with bradykinesia, and shuffling gait. The score on section III of the Unified Parkinson's Disease Rating Scale (UPDRS) before the administration of dopaminergic agents was 28. Tremor was not evident. He complained of memory impairment, especially of his short-term memory. He also occasionally had difficulty in looking for direction.
Initially his blood pressure was 120/80 mmHg, pulse rate was 78 beats/min, respiration rate was 20 breaths/min, and body temperature was 36.5℃. The results of complete blood cell count and serum biochemical analyses were normal. Thyroid function test, vitamin B12, and folate level were within normal limits. The results of a routine cerebrospinal fluid (CSF) investigation were normal. The adenosine deaminase level in the CSF was 1 IU/L. Cysticercus and other parasite-specific antibodies were not detected in the CSF, and other microbial parameters of the CSF were normal.
The scores on the Korean version of the Mini-Mental Status Examination, the Clinical Dementia Rating, and the Global Deterioration Scale were 10, 1, and 3, respectively. A neuropsychological test disclosed abnormal visuospatial function including in the interlocking-pentagons test, Rey figure-copying test, and memory domain (Table 1).
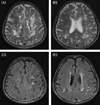
Plain films of the cervical-thoracic-lumbar spine showed no abnormalities. Brain MRI showed widespread multiple cystic dilated PVSs in the periventricular white matter and subcortical areas of both frontoparietal, left temporal, and left occipital lobes. Hyperintense signals were evident in white matter adjacent to the dilated PVSs on T2-weighted images and fast fluid-attenuated inversion-recovery images (Fig. 1). Magnetic resonance angiography produced normal results.
The patient was treated with levodopa (Sinemet® (100/25) at 150 mg/day) and bromocriptine (Antilactin® at 10 mg/day) for 2 months, but his condition did not improve. This treatment regimen was replaced with levodopa (Sinemet® at 300 mg/day), ropinirole (Requip® at up to 6 mg/day), and entacapone (Comtan® at 400 mg/day). After 6 months, bradykinesia and gait disturbance were moderately improved, and the score on section III of the UPDRS had improved to 22. Galantamine (Reminyl® at 8 mg/day) was administered at the initial stage, but this was stopped after 2 weeks due to drug-associated confusion without improvement, and further medication for dementia was not given due to the patient's refusal.
The PVSs of the brain are normal structures lined by pia that accompany arteries and arterioles as they penetrate the brain, and they are filled with ISF rather than CSF.1 They produce typical MRI findings. They are as small as 1.2 mm in diameter with a round, oval, or curvilinear shape. They have a well-defined and smooth margin along the path of penetrating arteries, and are isointense relative to CSF without contrast enhancement on MRI. If PVSs are dilated, they can be detected on thin-section T2-weighted MRI in up to 60% of healthy subjects.4 However, reports of disseminated polycystic dilated PVSs are rare.
PVS dilation occurs primarily in the elderly, and it has been considered as a normal aging process or a normal variant.5 The pathophysiology of abnormal dilatation of PVSs is unknown, but several mechanisms have been suggested. These include mechanical trauma from CSF pulsation or vascular ectasia, fluid exudation as a result of abnormalities of vessel-wall permeability, and ischemic perivascular tissue injury causing a secondary ex vacuo effect. Other possibilities include difficulty of brain interstitial fluid draining into the ventricles resulting from escalated intraventricular CSF pressure or lymphatic drainage obstruction of the brain, spiral elongation of the penetrating blood vessels, and interstitial fluid reabsorption.6-9 Also, dilated PVSs have been associated with aging, dementia, incidental white matter lesions, hypertension, and other vascular risk factors.1,4
Typically PVSs can be found in all parts of brain, but are most common in basal ganglia around the anterior commissure through the lenticulostriate artery. Less commonly, they are found in cerebral cortex, white matter, subinsular region, dentate nuclei, and cerebellum. Dilated or extremely enlarged PVSs are commonly found in the mesencephalothalamic region in territory of the paramedial mesencephalothalamic artery and in cerebral white matter, which may be related to the two layers of pia present in this location. Dilated PVSs in the mesencephalothalamic region may sometimes accompany obstructive hydrocephalus.
Dilated PVSs have many differential diagnoses, such as cystic neoplasms, parasitic cystic infection, ventricular diverticula, cystic infarction, non-neoplastic neuroepithelial cysts, and deposition disorder such as mucopolysaccharidosis.
Cysts and ventricular diverticula usually border the ventricle or subarachnoid space. Arachnoid cysts are extraparenchymal, and neuroepithelial cysts are not usually polycystic. In cystic neoplasm and non-neoplastic neuroepithelial cysts, the cysts may not be isointense with CSF on all MRI sequences. Also, cystic neoplasm is usually a single lesion and is found in the pons, cerebellum, and thalamus. Therefore, these diseases can be excluded based on MRI findings. Chronic infarct with cystic change is known to be isointense relative to CSF, and mucopolysaccharidosis also may be associated with dilated PVSs. However, these diseases present with different clinical findings and courses. Parasitic cysts, such as neurocysticercosis, are often present with a scolex. We could not find any scolex or serologic evidence of parasite infection in our patient.1,3,11
Most typical PVSs are asymptomatic, and hence are generally only incidentally found on MRI or at autopsy. Dilated PVSs can also be asymptomatic, or may develop various clinical manifestations. The most common presenting symptoms are nonspecific, such as headache and dizziness. Other symptoms include poor concentration, memory impairment, dementia, visual changes, oculomotor abnormality, tremor, tic, syncope, seizure, limb weakness, extrapyramidal symptoms, and ataxia.1,2
Multiple small dilated PVSs have been found previously in Parkinsonism patients, but their contribution to parkinsonian symptoms is still controversial. Some reports have suggested that multiple small dilated PVSs are responsible for Parkinsonism or modify the expression of coincidental Parkinsonism.12 In our patient the polycytic dilated PVSs were disseminated in both hemispheres, especially in the subcortical region including basal ganglia. Therefore, Parkinsonism in our patient may have developed from basal ganglia pathology or interruption of corticostriatal fibers, with dementia resulting from diffuse interruption of the subcortical-cortical connection. The structural damage resulted in a poor response to medication.
In cases of dilated PVSs with progressive symptoms and signs, especially those of huge size combined with hydrocephalus, surgical treatment is recommended. These include CSF diversion, ventriculoperitoneal shunt, cystoperitoneal shunt, ventriculocisternostomy, and implantation of a cyst catheter and reservoir.13 In the present case, a surgical approach was not possible due to the presence of multiple dilated PVSs that were disseminated in both hemispheres. Therefore, we treated our patient with anticholinesterase and dopaminergic agents. The outcome was sustained cognitive impairment and parkinsonian features with moderate symptomatic improvement. Surgery might be considered in our patient if his symptoms progress.
Figures and Tables
Figure 1
Clusters of variable-sized CSF-intensity cysts with minimal surrounding gliosis and expansion of overlying gyri were present in periventricular white matter and subcortical areas of both frontoparietal, left temporal, and left occipital lobes. Clusters of variable-sized cysts showed a high signal intensity on T2-weighted images (A, B) and a low signal intensity on fluid-attenuated inversion-recovery images (C, D).
References
1. Salzman KL, Osborn AG, Hause P, Jinkins JR, Ditchfield A, Copper JA, et al. Giant tumefactive perivascular spaces. Am J Neuroradiol. 2005. 26:298–305.
2. Maclullich AM, Wardlaw JM, Ferguson KJ, Starr JM, Seckl JR, Deary IJ. Enlarged perivascular spaces are associated with cognitive function in healthy elderly men. J Neurol Neurosurg Psychiatry. 2004. 75:1519–1523.

3. Rohlfs J, Riegel T, Khalil M, Iwinska-Zelder J, Mennel HD, Bertalanffy H, et al. Enlarged perivascular spaces mimicking multicystic brain tumors. Report of two cases and review of the literature. J Neurosurg. 2005. 102:1142–1146.
4. Patankar TF, Mitra D, Varma A, Snowden J, Neary D, Jackson A. Dilation of the Virchow-Robin space is a sensitive indicator of cerebral microvascular disease: study in elderly patients with dementia. Am J Neuroradiol. 2005. 26:1512–1520.
5. Barkhof F. Enlarged Virchow-Robin spaces: do they matter? J Neurol Neurosurg Psychiatry. 2004. 75:1516–1517.

6. Derouesne C, Gray F, Escourolle R, Castaigne P. 'Expanding cerebral lacunae' in a hypertensive patient with normal pressure hydrocephalus. Neuropathol Appl Neurobiol. 1987. 13:309–320.

7. Homeyer P, Cornu P, Lacomblez L, Chiras J, Derouesne C. A special form of cerebral lacunae: expanding lacunae. J Neurol Neurosurg Psychiatry. 1996. 61:200–202.

8. Longatti PL, Fiorindi A, Carteri A, Caroli F, Martinuzzi A. Expanding cerebral cysts (lacunae): a treatable cause of progressive midbrain syndrome. J Neurol Neurosurg Psychiatry. 2003. 74:393–394.

9. Poirier J, Barbizet J, Gaston A, Meyrignac C. Thalamic dementia. Expansive lacunae of the thalamo-paramedian mesencephalic area. Hydrocephalus caused by stenosis of the aqueduct of Sylvius. Rev Neurol (Paris). 1983. 139:349–358.
10. Heier LA, Bauer CJ, Schwartz L, Zimmerman RD, Morgello S, Deck MD. Large Virchow-Robin spaces: MR-clinical correlation. Am J Neuroradiol. 1989. 10:929–936.
11. Ozturk MH, Aydingoz U. Comparison of MR signal intensities of cerebral perivascular (Virchow-Robin) and subarachnoid spaces. J Comput Assist Tomogr. 2002. 26:902–904.

12. Fenelon G, Gray F, Wallays C, Poirier J, Guillard A. Parkinsonism and dilatation of the perivascular spaces (etat crible) of the striatum: a clinical, magnetic resonance imaging, and pathological study. Mov Disord. 1995. 10:754–760.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download