Abstract
Purpose
Bacillus Calmette-Guérin (BCG) vaccine has widely been used to immunize against tuberculosis, but its protective efficacy is variable in adult pulmonary tuberculosis, while it is not efficiently protective against progressive infection of virulent Mycobacterium tuberculosis strains. In this study, the protective effects of plasmid DNA vaccine constructs encoding IL-12 or IL-18 with the BCG vaccine were evaluated against progressive infection of M. tuberculosis, using mouse aerosol challenge model.
Materials and Methods
Plasmid DNA vaccine constructs encoding IL-12 or IL-18 were constructed and mice were immunized with the BCG vaccine or with IL-12 DNA or IL-18 DNA vaccine constructs together with the BCG vaccine.
Results
The BCG vaccine induced high level of interferon gamma (IFN-γ) but co-immunization of IL-12 or IL-18 DNA vaccine constructs with the BCG vaccine induced significantly higher level of IFN-γ than a single BCG vaccine. The BCG vaccine was highly protective at early stage of M. tuberculosis infection, but its protective efficacy was reduced at later stage of infection. The co-immunization of IL-12 DNA vaccine constructs with the BCG vaccine was slightly more protective at early stage of infection and was significantly more protective at later stage infection than a single BCG vaccine.
Tuberculosis (TB) has a long history with human beings, and it is still considered to be a major infectious disease in the world. One third of the world's population is infected with Mycobacterium tuberculosis, a cause of TB, which causes high morbidity and mortality.1,2 Current situation has been worsened by co-infection with human immunodeficiency virus and by emergence of multi-drug resistant or extensively-drug resistant M. tuberculosis strains. M. bovis bacillus Calmette-Guérin (BCG) has been utilized worldwide as an effective anti-TB vaccine,3 however, its protective efficacy is variable, ranging from 0 to 80%, against pulmonary TB.4 Furthermore, the BCG vaccine-induced immune responses decrease gradually three months after vaccination in humans.5 In animal model, the BCG vaccine is protective against M. tuberculosis challenge, but the protective efficacy of the BCG vaccine decreases 6-12 months post-immunization.6-8 The BCG vaccine is protective at early stage of infection against several M. tuberculosis strains, but can not efficiently protect the progression of M. tuberculosis infection, especially against highly virulent M. tuberculosis strains.9,10
Cytokines play a key role in cell-mediated immune responses because they are able to modulate the immune response and enhance the protective immune response. Interferon gamma (IFN-γ) is a typical T helper 1 (Th1) cytokine that is secreted mainly by cluster of differentiation 4 (CD4) and CD8 T-cells,11 and has been known as a key cytokine for protective immunity against TB.12 IL-12 and IL-18 are representative cytokines produced by activated macrophages, dendritic cells and natural killer (NK) cells, that promote the production of IFN-γ of T-cells.13-15 IL-12 or IL-18 has shown good adjuvant effects on the BCG vaccine, protein subunit vaccine, and plasmid DNA vaccine.14,16-20
In this study, we investigated whether co-immunization of plasmid DNA vaccine constructs, encoding IL-12 or IL-18, with the BCG vaccine could enhance the protective immunity of the BCG vaccine and efficiently protect the progression of M. tuburculosis infection, using an aerosol challenge mouse model.
Specific pathogen-free female C57BL/6 mice at 5-6 weeks of age were purchased from Japan SLC, Inc. (Shijuoka, Japan), maintained under barrier conditions in a biohazard animal room at Yonsei University Medical Research Center, and fed a sterile commercial mouse diet and water. All animal experiments were done according to the regulation of Institutional Animal Care and Use Committee, Yonsei University Health System.
Mycobacterium tuberculosis H37Rv (ATCC 27294) and M. bovis BCG (Pasteur strain 1173P2) used in this study were prepared as previously described.21 Briefly, M. tuberculosis and M. bovis BCG were grown for about 10 days at 37℃ as a surface pellicle on Sauton medium enriched with 0.4% sodium glutamate and 3.0% glycerine. The surface pellicles were collected and disrupted with 6 mm glass beads by gentle vortexing. After clumps were settled down, the upper suspension was collected and aliquots were stored at -70℃ until use. After thawing, viable organisms were then counted by plating serial dilutions on the Middlebrook 7H11 agar (Difco, Detroit, MI, USA). For inoculation into mice, bacterial suspensions were sonicated briefly in a sonic bath and diluted with phosphate-buffered saline (PBS) to reach desired bacilli numbers.
Culture filtrate protein antigens (CFP) were produced from the culture of M. bovis BCG as previously described.21 Briefly, M. bovis BCG was grown in Sauton medium on an orbital shaker for 7 days. The supernatants were sterile filtered and concentrated via ultrafiltration over an Amicon ultrafiltration stirred cell (Amicon, Danvers, MA, USA) fitted with a PM10 membrane (Millipore, Bedford, MA, USA). Samples were further concentrated to a final volume of about 5 mL by centrifugation in a Savant speed-Vac (Savant Instruments, Holbrook, NY, USA). These preparations were aliquoted in 1 mL samples and stored at -20℃. Residual endotoxin levels were <50 IU/mg as determined by a Limulus amebocyte lysate assay (Cambrex, East Rutherford, NJ, USA).
The vector pGX10 expressing IL-12 or IL-18 was prepared as described previously.16,22 Briefly, pGX10, plasmid DNA vector, was generated by replacing the ampicillin resistance cassette of pTV2 with the kanamycin resistance cassette. pGX10 is composed of a cytomegalovirus (CMV) immediate-early gene promoter, tripartite leader (TPL) sequences, simian virus 40 (SV40) late polyA tail, and an SV40 enhancer, linked in tandem. For generation of pGX10-IL-12, murine IL-12p35 (p35), the internal ribosomal entry site (IRES) of encephalomyocarditis virus, and murine IL-12p40 (p40) were also linked in a tandem, unidirectionally arranged and inserted into pGX10 vector, resulting in pGX10-p35/IRES/p40 (pGX10-IL-12) (Fig. 1). For generation of pGX10-IL-18, murine IL-18 cDNA was provided kindly from Bank for Cytokine Research at Chonbuk University. IL-18 cDNA was inserted into pGX10 vector (Fig. 1). pGX10, pGX10-IL-12, and pGX10-IL-18 plasmid DNA for immunization were prepared by cesium chloride density gradient centrifugation which was performed at 50,000 rpm for 15 h at 20℃ using a Beckman LE80 ultracentrifuge.22
Mice were immunized with experimental vaccines as previously described.22 Mice were given subcutaneously a single dose (2×105 CFU/mouse) of the BCG vaccine in a volume of 200 µL on the back. For DNA vaccination, mice were injected twice at 3-weeks intervals intramuscularly into the quadriceps muscle with 50 µg of pGX10, pGX10-IL-12 or pGX10-IL-18 DNA vaccine constructs in a volume of 200 µL. For the co-immunization of plasmid DNA vaccine constructs and the BCG vaccine, mice were injected together subcutaneously with the BCG vaccine and simultaneously with the plasmid DNA intramuscularly at the first immunization, and then were injected intramuscularly with plasmid DNA on the third week after the first immunization. For negative controls, mice were injected subcutaneously with 200 µL of sterile pyrogen-free saline or, intramuscularly with 50 µg of pGX10, pGX10-IL-12, and pGX10-IL-18 DNA vaccine constructs.
Mycobacterial antigen-specific immune responses were measured as previously described.21 Mice were sacrificed on the fourth week after last immunization, and spleens were removed from three mice in each experimental group. Splenocytes were obtained by preparing single-cell suspension from spleens by dispersion of the tissue with sterilized slide glasses. Erythrocytes were lysed with a solution containing 155 mM ammonium chloride and 10 mM potassium bicarbonate buffer, and cells were thoroughly washed. Isolated cells were cultured in 96-well cell culture plates (Nunc, Roskilde, Denmark), each well containing 2×105 lymphocytes in a 200 µL volume of RPMI 1640 supplemented with 5×10-5 M 2-mercaptoethanol, 1% penicillin-streptomycin, 1 mM glutamine, and 10% (vol/vol) fetal calf serum. The splenocytes were stimulated in triplicate culture with CFP at final concentration of 10 µg/mL or with medium only. The splenocytes were incubated at 37℃ in the presence of 5% CO2 for 6 days, the supernatants were taken, and IFN-γ present in culture supernatants was quantified using a mouse IFN-γ OptEIA™ Set (Pharmingen, San Diego, CA, USA) according to the manufacturer's instructions. In brief, enzyme immunoassay (EIA) plates (Costar, Cambridge, MA, USA) were coated overnight with 2 µg/mL monoclonal anti-mouse IFN-γ in polycarbonate buffer (pH9.2) at 4℃. Free binding sites were blocked with PBS containing 0.05% Tween 20 (PBST) and 5% normal goat serum, followed by washing with PBST. Culture superna-tants of day 6 for IFN-γ were added into wells in triplicate, and the plate was then incubated overnight at 4℃. The recombinant mouse IFN-γ was used as a standard. The wells were then washed four times with PBST, and IFN-γ was detected with a biotin-labeled rat anti-mouse IFN-γ monoclonal antibody and phosphate-conjugated streptoavidin. After six times washing with PBST, the enzyme reaction was developed with O-phenylenediamine (Sigma-Aldrich Chemical Co., St. Louis, MO, USA) and hydrogen peroxide. Optical density was measured by an automatic EIA reader (Molecular Devices Co., Sunnyvale, CA, USA) after stopping the reaction by adding 2.5 N H2SO4, and the quantity of IFN-γ was calculated by comparing with the results obtained with its standard.
Mice were challenged by aerosol exposure with M. tuberculosis H37Rv using an inhalation device (Glas-Col, Terre Haute, IN, USA) which was calibrated to deliver approximately 200 bacteria into the lungs as previously described.10,22 Mice were challenged on the fourth week after the last immunization, and five mice per group were sacrificed at 30 and 90 days post-challenge and bacterial counts were determined from tissue homogenates of the lung and spleen. The numbers of viable bacteria in the lung and spleen were determined by plating with serial dilution of whole organ homogenates on Middlebrook 7H11 agar (Difco, Detroit, MI, USA). Colonies were counted after 3-4 weeks incubation at 37℃. The resulting values are based on the experimental group of five mice and are presented as means of log10CFU±standard deviation per group.
Lungs were excised from all animals and stored in 10% formalin, and then embedded and stained with haematoxylin and eosin (H&E) for pathological analysis. The level of inflammation in the lungs was evaluated as previously described.10 In brief, H&E stained lung sections were photographed using a microscope (Olympus BX51, Olympus Co., Tokyo, Japan), and the level of inflammation present in the images was analyzed using the ImageJ program (National Institutes of Health, MD, USA). The resulting values are presented as the mean percent of inflamed area from lung sections of five mice per group.
Production of IFN-γ, a key cytokine of the protective immune response against TB, was examined to investigate whether DNA vaccines encoding IL-12 or IL-18 cytokine were able to augment the protective immune response of the BCG vaccine. Thus, splenocytes from three mice from each group were isolated after four weeks of last immunization and were stimulated with CFP or media for 6 days, and IFN-γ levels in the supernatants were measured. The level of CFP-reactive IFN-γ in the BCG vaccinated mice was approximately 2,000 pg/mL (Fig. 2). The levels of CFP-reactive IFN-γ in the groups co-immunized with the BCG vaccine and IL-12 or IL-18 DNA vaccine were approximately 3,000 pg/mL and 4,600 pg/mL, respectively (Fig. 2), which were significantly higher than that in the BCG vaccine (p<0.01 and p<0.001, IL-12 and IL-18 DNA vaccine constructs, respectively). The levels of CFP-reactive IFN-γ in the pGX10 vector, IL-12 and IL-18 DNA vaccine constructs-immunized mice were below 200 pg/mL as naïve mice (data not shown).
To evaluate the effect of the plasmid DNA vaccine constructs encoding cytokines on the protective efficacy of the BCG vaccine, ten immunized mice per group were challenged by aerosol exposure with a virulent strain of M. tuberculosis, and the protective efficacy of the vaccine candidates was measured by comparing the bacterial burdens in the organs of vaccinated mice to those of naïve mice (Fig. 3).
The protective efficacy was measured at 30 days post-infection, which is the point when M. tuberculosis bacilli undergoes exponential growth and reaches peak in the lungs. The bacterial CFUs in naïve mice at 30 days post-infection were 6.37±0.13 log and 5.41±0.04 log in the lungs and spleens, respectively. There were significant bacterial reductions in the BCG vaccinated mice [1.1 log (p<0.001) in the lungs and 0.67 log (p<0.01) in the spleens, respectively] compared to naïve control group. There were no significant bacterial reductions in the IL-12 or IL-18 DNA vaccine-immunized mice (data not shown). There was a significant bacterial reduction not only in the lungs but also in the spleens of mice co-immunized with IL-12 DNA vaccine and the BCG vaccine (0.3 log, p<0.05; in the spleen). However, there was no significant bacterial reduction in the mice co-immunized with IL-18 DNA vaccine, and the BCG vaccine.
To examine whether IL-12 or IL-18 DNA vaccine construct helps the BCG vaccine prevent the progression of M. tuberculosis infection, mice were sacrificed and bacterial CFUs were measured at 90 days post-infection (Fig. 3). The CFUs in the naïve mice were 6.13 log and 5.62 log in the lungs and spleens, respectively, at 90 days post-infection. The CFUs in the BCG vaccine-immunized mice were increased at 90 days post-infection (5.76 log and 5.00 log in the lungs and spleens, respectively) compared to CFUs at 30 days post-infection. The protective efficacy of the BCG vaccine was reduced to 0.37 log (p<0.05) in the lungs at 90 days post-infection. There was no significant protective efficacy in the IL-12 or IL-18 DNA vaccine-immunized mice at 90 days post-infection (data not shown).
Although CFUs in the mice co-immunized of with IL-12 DNA vaccine and the BCG vaccine was increased at 90 days post-infection compared to at 30 days post-infection, this vaccine induced high significant protective efficacy in the lungs and spleens at 90 days post-infection (p<0.001 both lungs and spleens). Interestingly, however, there were significant bacterial reductions in the lung (0.30, p<0.05) and spleen (0.47 log, p<0.01) compared to the BCG vaccinated mice. The co-immunization of IL-18 DNA vaccine with the BCG vaccine induced significant protective efficacy at 90 days post-infection and there was a little reduction of CFUs in the spleens compared to the BCG vaccinated mice, but it was not significant.
The level of lung inflammation was assessed to evaluate the effectiveness of IL-12 or IL-18 DNA vaccine constructs on the BCG vaccine. Thus, mice were sacrificed at 30 and 90 days post-infection and five H&E stained lung sections per group were analyzed using the ImageJ software. Representative lung sections and the lung inflammation values are shown in Fig. 4 and Table 1. In the lungs of naïve mice, considerable and consolidated inflammation was seen, and the mean inflammation values were 38% and 43% at 30 and 90 days post-infection, respectively. In the BCG vaccinated mice, less inflammation was observed, and the mean inflammation values were significantly lower compared to naïve group (p<0.001 and p<0.01, at 30 and 90 days post-infection, respectively). In the vector DNA, IL-12 DNA, or IL-18 DNA vaccine constructs-immunized mice, the levels of inflammation in the lungs were similar to that of naïve mice group. In the co-immunized mice of IL-12 DNA or IL-18 DNA vaccine constructs with the BCG vaccine, lung inflammations were not improved at 30 days post-infection, but interestingly, the less lung inflammation was observed in the co-immunized mice of IL-12 DNA and the BCG vaccine at 90 weeks post-infection compared to the BCG vaccinated mice (p<0.05).
The BCG vaccine induces cellular immune responses, and CD4 T-cell is major protective machinery against TB, which secretes cytokines such as IFN-γ and TNF-α resulting in the activation of macrophages to kill infected M. tuberculosis. Antigen-presenting cells such as macrophages or dendritic cells, reversely, can enhance the functions of T cell immune machinery. Th1 cytokines such as IL-12 or IL-18, secreted by macrophages or dendritic cells, can act as an adjuvant to moderate immune responses of T-cells.14,16,19
In the present study, IL-12 or IL-18 cytokines in the form of plasmid DNA were evaluated as an adjuvant of the BCG vaccine against progressive TB. Co-immunization of IL-12 or IL-18 DNA vaccine constructs with the BCG vaccine could enhance IFN-γ production compared to the BCG vaccine alone. These results are consistent with the previous reports: Palendira, et al.'s reported that IL-12 DNA vaccine constructs induced plasmid DNA encoding mycobacterial antigen to produce the Th1 immune response,18,19 and Triccas, et al.20 showed that IL-18 DNA vaccine constructs augmented IFN-γ production of the DNA vaccine encoding TB antigen. These results suggest that IL-12 or IL-18 DNA vaccine constructs have a synergistic effect on the protective immune response by the BCG vaccine.
In the present study, the BCG vaccine induced highly significant protective efficacy against TB in the early stage of infection, and the co-immunization of IL-12 DNA vaccine constructs with the BCG vaccine showed a little additive effect on the BCG vaccine in the spleens. This result is consistent with Palendira, et al.'s report14,19 that IL-12 DNA vaccine constructs induced the BCG vaccine to reduce the bacterial burden than a single BCG vaccine.
The protective efficacy of the BCG vaccine was reduced at the later stage of infection. This result is consistent with the previous reports.10,23 Interestingly, the co-immunization of IL-12 DNA vaccine constructs with the BCG vaccine induced more protective efficacy than the BCG vaccine at later stage of infection, and this vaccine efficiently inhibited the growth of M. tuberculosis in the spleens. The level of pulmonary inflammation in the coimmunized mice of IL-12 DNA vaccine constructs with the BCG vaccine was also significantly lower than the BCG vaccine at later stage of infection. However, the co-immunization of IL-18 DNA vaccine constructs with the BCG vaccine showed no additive effects on the protective efficacy of the BCG vaccine, even though the high level of IFN-γ was induced by co-immunization of IL-18 DNA vaccine constructs with the BCG vaccine. This suggests that IFN-γ production did not translate into protective efficacy against M. tuberculosis challenge, according to the results of in vitro assays. It was previously demonstrated that despite the increase of IFN-γ production by T-cells, co-immunization with IL-18 DNA vaccine constructs did not augment the protective efficacy of M. tuberculosis Ag85B DNA vaccine constructs.20 Similar observations were also noticed in the case of IL-18 against Brucella abortus 544 challenge and respiratory syncytial virus infec-tion.24,25 This could be explained by the fact that IL-18 induces IFN-γ and also recruits other immune cells such as NK cells and neutrophils. IL-18 induced IFN-γ production as well as weight loss during respiratory syncytial virus infection, which is related to the recruitment of activated NK cells in the lungs and airwary by IL-18.25
These results presented in this study suggest that co-immunization of IL-12 with the BCG vaccine enhances the protective immune response of the BCG vaccine and augments the BCG vaccine to protect efficiently against progressive TB.
Figures and Tables
Fig. 1
Construction of plasmid DNA encoding IL-12 and IL-18. Plasmid DNA vector, pGX10, is composed of a CMV promotor, TPL, SV40 late polyA tail, and an SV40 enhancer. pGX10-IL-12 was generated by insertion of murine IL-12p35, the internal ribosomal entry site (IRES) of encephalomyocarditis virus, and murine IL-12p40. pGX10-IL-18 was generated by insertion of murine IL-18. SV40, simian virus 40; pCMV, CMV promoter; TPL, tripartite leader sequences; CMV, cytomegalovirus.

Fig. 2
IFN-γ production from splenocytes by experimental vaccines. Spleno-cytes were prepared from three mice per group 30 days after the last vaccination and then stimulated with CFP antigens in vitro. The level of IFN-γ after 6 days of incubation was analyzed. The experiments were repeated three times with similar results, and the data from one representative experiment are shown. The results are expressed as the mean amounts of IFN-γ (pg/mL) of three mice (±SD) per group. p-value less than 0.05 was considered to be significant and was represented as follows: IFN-γ, interferon gamma; CFP, culture filtrate protein antigens; BCG, Bacille-Calmette-Guerin. *p<0.05, **p<0.001.

Fig. 3
Protective efficacy of experimental vaccines. Ten mice per group were immunized with the BCG vaccine singly, and co-immunized with the BCG vaccine and IL-12 DNA or IL-18 DNA vaccine. Immunized mice were infected by aerosol exposure with virulent M. tuberculosis H37Rv. The number of CFUs was measured in the lungs (A) and spleens (B) of five mice per group at 30 and 90 days post-challenge. The level of protection of experimental vaccines was calculated as CFUs in the naïve minus CFUs in the vaccinated mice. Data are represented as log10 CFU±SD, where n=5 and data is representative of two experiments. The ANOVA test was used to determine the significance of differences between experimental groups. p-value less than 0.05 was considered as significant and represented as follows; *p<0.05, **p<0.01, and ***p<0.001. BCG, bacillus Calmette-Guérin; CFU, colony forming unit; ANOVA, analysis of variance.

Fig. 4
Histopathology of lungs after aerosol infection with virulent M. tuberculosis. The right middle lobe of the lungs of mice was removed after 90 days post-challenge and lung sections were stained with H&E (×20). The representative histopathology of lungs of naïve mice (A), the BCG vaccine-immunized mice (B), co-immunized mice of the BCG vaccine and IL-12 DNA vaccine (C), and IL-18 DNA vaccine (D).
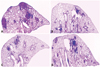
Table 1
Lung Inflammation Values in Naïve and Vaccinated Mice at 30 and 90 days Post-Infection with M. tuberculosis H37Rv Strain

Significantly reduced lung inflammation in the vaccinated mice relative to naïve control is indicated as follows: *p<0.01, **p<0.001.
†Lung inflammation values are presented as the mean percentage of the area of inflammation from lung lections of five mice per group (±standard deviation).
‡Lung inflammation value of coimmunized mice of IL-12 DNA vaccine with BCG vaccine is significantly lower than that of BCG-vaccinated mice at 12 weeks post-infection (p<0.05).
ACKNOWLEDGEMENTS
This work was supported by the international Research & Development Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (MEST), Republic of Korea (Grant number: K21001001623-10B1300-03110, SNC), and by a grant from Brain Korea 21 Project for Medical Science in Yonsei University (SNC).
References
1. Raviglione MC, Snider DE Jr, Kochi A. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA. 1995. 273:220–226.

2. Snider DE Jr, Castro KG. The global threat of drug-resistant tuberculosis. N Engl J Med. 1998. 338:1689–1690.

3. Hawgood BJ. Albert Calmette (1863-1933) and Camille Guérin (1872-1961): the C and G of BCG vaccine. J Med Biogr. 2007. 15:139–146.

4. Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995. 346:1339–1345.

5. Weir RE, Gorak-Stolinska P, Floyd S, Lalor MK, Stenson S, Branson K, et al. Persistence of the immune response induced by BCG vaccination. BMC Infect Dis. 2008. 8:9.
6. Derrick SC, Yang AL, Morris SL. A polyvalent DNA vaccine expressing an ESAT6-Ag85B fusion protein protects mice against a primary infection with Mycobacterium tuberculosis and boosts BCG-induced protective immunity. Vaccine. 2004. 23:780–788.

7. Olsen AW, Brandt L, Agger EM, van Pinxteren LA, Andersen P. The influence of remaining live BCG organisms in vaccinated mice on the maintenance of immunity to tuberculosis. Scand J Immunol. 2004. 60:273–277.

8. Weinrich Olsen A, van Pinxteren LA, Meng Okkels L, Birk Rasmussen P, Andersen P. Protection of mice with a tuberculosis subunit vaccine based on a fusion protein of antigen 85b and esat-6. Infect Immun. 2001. 69:2773–2778.

9. Grode L, Seiler P, Baumann S, Hess J, Brinkmann V, Nasser Eddine A, et al. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guérin mutants that secrete listeriolysin. J Clin Invest. 2005. 115:2472–2479.

10. Jeon BY, Derrick SC, Lim J, Kolibab K, Dheenadhayalan V, Yang AL, et al. Mycobacterium bovis BCG immunization induces protective immunity against nine different Mycobacterium tuberculosis strains in mice. Infect Immun. 2008. 76:5173–5180.
11. Lee J, Hartman M, Kornfeld H. Macrophage apoptosis in tuberculosis. Yonsei Med J. 2009. 50:1–11.
12. Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993. 178:2249–2254.

13. Emoto M, Emoto Y. Intracellular bacterial infection and invariant NKT cells. Yonsei Med J. 2009. 50:12–21.

14. Jeong E, Lee JY. Intrinsic and extrinsic regulation of innate immune receptors. Yonsei Med J. 2011. 52:379–392.

15. Kinjo Y, Kawakami K, Uezu K, Yara S, Miyagi K, Koguchi Y, et al. Contribution of IL-18 to Th1 response and host defense against infection by Mycobacterium tuberculosis: a comparative study with IL-12p40. J Immunol. 2002. 169:323–329.

16. Ha SJ, Park SH, Kim HJ, Kim SC, Kang HJ, Lee EG, et al. Enhanced immunogenicity and protective efficacy with the use of interleukin-12-encapsulated microspheres plus AS01B in tuberculosis subunit vaccination. Infect Immun. 2006. 74:4954–4959.

17. Luo Y, Yamada H, Chen X, Ryan AA, Evanoff DP, Triccas JA, et al. Recombinant Mycobacterium bovis bacillus Calmette-Guén (BCG) expressing mouse IL-18 augments Th1 immunity and macrophage cytotoxicity. Clin Exp Immunol. 2004. 137:24–34.

18. Martin E, Kamath AT, Briscoe H, Britton WJ. The combination of plasmid interleukin-12 with a single DNA vaccine is more effective than Mycobacterium bovis (bacille Calmette-Guérin) in protecting against systemic Mycobacterim avium infection. Immunology. 2003. 109:308–314.

19. Palendira U, Kamath AT, Feng CG, Martin E, Chaplin PJ, Triccas JA, et al. Coexpression of interleukin-12 chains by a self-splicing vector increases the protective cellular immune response of DNA and Mycobacterium bovis BCG vaccines against Mycobacterium tuberculosis. Infect Immun. 2002. 70:1949–1956.
20. Triccas JA, Sun L, Palendira U, Britton WJ. Comparative affects of plasmid-encoded interleukin 12 and interleukin 18 on the protective efficacy of DNA vaccination against Mycobacterium tuberculosis. Immunol Cell Biol. 2002. 80:346–350.

21. Jeon BY, Kim HJ, Kim SC, Jo EK, Park JK, Paik TH, et al. Protection of mice against Mycobacterium tuberculosis infection by immunization with aqueous fraction of Triton X-100-soluble cell wall proteins. Scand J Immunol. 2008. 67:18–23.
22. Ha SJ, Jeon BY, Kim SC, Kim DJ, Song MK, Sung YC, et al. Therapeutic effect of DNA vaccines combined with chemotherapy in a latent infection model after aerosol infection of mice with Mycobacterium tuberculosis. Gene Ther. 2003. 10:1592–1599.

23. Hinchey J, Lee S, Jeon BY, Basaraba RJ, Venkataswamy MM, Chen B, et al. Enhanced priming of adaptive immunity by a proapoptotic mutant of Mycobacterium tuberculosis. J Clin Invest. 2007. 117:2279–2288.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download