Abstract
In covering wounds, efforts should include utilization of the safest and least invasive methods with goals of achieving optimal functional and cosmetic outcome. The recent development of advanced wound healing technology has triggered the use of cells to improve wound healing conditions. The purpose of this review is to provide information on clinically available cell-based treatment options for healing of acute and chronic wounds. Compared with a variety of conventional methods, such as skin grafts and local flaps, the cell therapy technique is simple, less time-consuming, and reduces the surgical burden for patients in the repair of acute wounds. Cell therapy has also been developed for chronic wound healing. By transplanting cells with an excellent wound healing capacity profile to chronic wounds, in which wound healing cannot be achieved successfully, attempts are made to convert the wound bed into the environment where maximum wound healing can be achieved. Fibroblasts, keratinocytes, adipose-derived stromal vascular fraction cells, bone marrow stem cells, and platelets have been used for wound healing in clinical practice. Some formulations are commercially available. To establish the cell therapy as a standard treatment, however, further research is needed.
We all experience our fair share of wounds during the course of our lives. The art and science of wound healing are complex and intriguing. During the past few decades, various wound healing technologies for promoting cell activity or minimizing scar formation have been developed and some of them are being actively used at present. The aim of this paper is to provide information on cell-based treatment options in clinical setting for healing of acute and chronic wounds.
Wound healing is an intricate process whereby the skin (or another organ/tissue) repairs itself after injury. The skin is composed of three layers; the most external layer is the epidermis, the next layer is the dermis, and beneath the dermis lies the subcutaneous fat layer. In superficial or partial thickness wounds, where the damage is limited to the epidermis or the upper dermal layer, only the epidermis needs to be regenerated, resulting in quick healing and minimal scar formation. However, in serious wounds that penetrate deeper than the mid-dermal layer with skin avulsion or subcutaneous fat exposure, complications such as infection develop more frequently and scars tend to remain even after the wound is fully healed.
Wounds heal by repair and/or regeneration. There is a subtle distinction between 'repair' and 'regeneration'. Repair refers to the physiologic adaptation of an organ after injury in an effort to re-establish continuity without regard to the exact replacement of lost/damaged tissue. Regeneration refers to the replacement of lost/damaged tissue with an 'exact' copy, such that both morphology and functionality of the tissue are completely restored. The skin of mammals does not regenerate spontaneously, but heals with scars (repair).
Wounds heal according to a specific, sequenced process: 1) hemostasis (not considered a phase by some authors), 2) inflammation, 3) proliferation and 4) remodeling. Upon injury to the skin, a set of complex biochemical events takes place in a closely orchestrated cascade to repair the damage. The duration of the wound healing process in reality tends to vary across individuals and different severities of the wound.
Selecting an appropriate wound healing strategy according to the condition of the wound is crucial for successful wound healing, since it can minimize the risk of complications, enhance the speed of wound healing, and minimize scar formation after the wound has fully healed. A variety of methods have been used for wound healing. These include healing by primary intention, secondary intention, tertiary intention, skin grafts, and flaps (1-4).
In primary intention, wounds heal by the process of epithelialization. Primary intention healing can be applied for wounds involving the epidermis and dermis without total penetration of the dermis. When wound edges are brought adjacent to each other with sutures (stitches), staples, or adhesive tape (approximated wound), the wound also heals by primary intention. Well-repaired lacerations and most surgical wounds also heal by primary intention healing. Primary intention can minimize scarring, but the role of primary closure is limited because of the size and shape of the defect. Only small defects with elliptical shapes yield satisfactory results after primary closure.
In secondary intention, wounds heal by granulation formation (fibrosis), contraction, and epithelialization. Wound care must be performed to prevent infection and to encourage granulation tissue formation. Unrepaired full thickness open wounds heal by secondary intention. Healing by secondary intention usually leaves significantly conspicuous and unfavorable scars. Darker skin is more prone to hypertrophic scarring or keloid formation (5).
In tertiary intention (delayed primary closure or secondary suture), wounds are initially left open and closed after several days (typically 4 or 5 days) by approximation or by the use of tissue grafts (skin grafts or flaps). During the first 4 to 5 days, the wound is cleaned, debrided, and observed. This type of healing may be desired in cases of contaminated wounds. By the 4th or 5th day, phagocytosis of contaminated tissues takes place and the wound enters the proliferation phase. Usually, the wound is closed surgically at this juncture.
Skin grafting is a type of graft surgery involving the transplantation of skin. Skin graft does not have an intact blood supply and therefore relies on the growth of new blood vessels from the wound bed. Skin grafts are often employed to treat extensive wounds caused by trauma, burn, removal of skin cancer, and infection. Skin grafts serve two purposes: reduce the course of required treatment (and time in the hospital), and improve the function and appearance of the area of the body which receives the skin graft. Skin grafting is generally straightforward with a relatively low risk of complications. However, two major concerns regarding skin grafting are poor color matching in the recipient site and donor site morbidity, including pain, discomfort, and hypertrophic scarring. Major pigment mismatches are particularly common in dark-skinned patients, including Asians (6).
Skin and soft tissue injuries can be also treated using various types of flaps, including local, distant, island, and free flaps. Local flap can be a useful strategy for entire thickness skin and soft tissue defect. However, in certain cases, the use of a local flap is not feasible, primarily because of limitations of size and rotation arc. In such cases, an island or a free flap may also be applied (7-9). However, this surgical procedure often needs microsurgery facilities and techniques, takes longer, and is frequently associated with donor site morbidity (10, 11). Skin grafting or flap coverage can be also a burden to patients due to the involved surgical procedures, especially for elderly people in whom skin cancer is fairly common.
Each conventional repair method has advantages and limitations. Recently, the development of advanced wound healing technology has triggered the use of cells to overcome limitations of the conventional methods. Cell therapy has a potential to improve wound healing conditions without major surgical procedures and donor-site morbidity. Cell therapy can be applied for both acute and chronic wounds.
In the treatment of acute wounds, cell therapy can increase wound healing rate, reduce scar contracture, and minimize donor-site morbidity. This method also provides a tissue that is similar to the surrounding skin, leaving minimal scars and color mismatch. Since the epidermal portion can be restored by epithelialization induced by the migration and proliferation of adjacent epidermal cells including melanocytes, the density and activity of melanocytes as well as precursor melanocytes of the epidermis of the graft become similar to those observed in the adjacent skin. In addition, this technique is simple, less time-consuming, and reduces the surgical burden for patients.
In the treatment of chronic wounds, attempts are made to convert the wound bed into the environment where maximum wound healing can be achieved by transplanting cells with an excellent wound healing capacity to the wound bed.
Cell therapy can be used for the treatment of various types of skin defects including trauma, burn wounds, scar excision, leg ulcer, donor sites of split-thickness skin, and excision of skin tumor (12, 13). In particular, cell therapy can be useful for elderly patients in whom skin grafting or flap coverage can be a burden due to surgical procedures or patients who do not want to undergo major surgery. In addition, this method can be a good option in covering defects involving the face and upper extremities, in which efforts should include choosing the safest and least invasive method with a goal of achieving optimal functional and cosmetic outcomes.
Cell therapy can be also effective in the treatments of chronic wounds including diabetic ulcers. In cases of chronic wounds, wound healing cannot often be achieved successfully because of the involvement of multiple factors (14, 15). One of main contributing factors for the non- or delayed healing wound is decreased function of the key cells for the wound healing including fibroblasts and keratinocytes. In chronic wound patients these cells have notably decreased mitotic activity compared with normal healthy controls. Besides, there is an insufficient degree of synthesis of extracellular matrices and growth factors that are essential for wound healing. Conversely, secretion of matrix metalloproteinase which destroys tissue proteins becomes activated. The degree of cell migration is also decreased. Therefore, the wound remains open without being cured for long periods of time. This eventually leads to infections. It would therefore be mandatory to activate these cells during treatment. By transplanting cells with an excellent profile of wound healing capacity to the wound bed (the term may sound grand, but this method actually refers to the attachment or spraying the cells to the wound bed), attempts are made to convert the wound bed into the environment where maximum wound healing can be achieved.
Cell therapy using autologous cells accelerates the wound healing process by reducing the time needed for the host cells to invade the wound tissue and by early synthesis of new skin (16-18). Based on previous research, cell-only treatment accelerates the rate of wound healing, but does not show a beneficial effect on wound contraction (19, 20). Therefore, cells are usually used with artificial dermis as a tissue-engineered dermis to optimize wound healing. Cell-seeded artificial dermis graft minimizes wound contraction without delaying wound healing for the treatment of skin and soft tissue defects. Artificial dermis can be used alone without cells for wound coverage. However, in the absence of cell components, wounds treated with artificial dermis often show delayed healing and heal with altered patterns of scarring, resulting in scar contractures (21, 22).
Cell therapy using allogeneic cells has also impressive therapeutic value in wound healing (23, 24). Initially there was some confusion as to how the wound-healing effects of allogeneic cells were exerted. It became clear that allogeneic cells do not attach and cover the wound permanently and are soon replaced by host cells. However, allogeneic cells promote migration and proliferation of host cells from the wound beds and edges because allogeneic cells release not only growth factors, but also extracellular matrices and basement membrane components. Eventually, the role of allogeneic cells in the wound healing process is to accelerate epithelialization from the wound edges and promote granulation formation from wound beds.
Procedure of cell therapy is different depending on cells. At present, keratinocytes, fibroblasts, and adipose-derived stromal vascular fraction (SVF) cells are actively used in the clinical setting. To culture fibroblasts and keratinocytes for clinical use, a skin biopsy of approximately 1 cm2 is taken from a patient and sent to a commercial laboratory. In Korea, the Korean Food and Drug Administration (FDA) have approved two commercial laboratories for fibroblast culture: S Biomedics (Seoul, Korea) and ChaBio & Diostec (Seoul, Korea) and one for keratinocyte culture: Tego Science (Seoul, Korea). Briefly, cells are isolated using enzyme and propagated. SVF cells, which can be obtained from adipose tissue, are relatively easy to harvest in large quantities without cell cultures. Briefly, abdominal adipose tissues are harvested from a patient by liposuction. The obtained samples are rinsed and then incubated in cell culture medium containing collagenase. The top lipid layer is removed and the remaining liquid portion is centrifuged. The resulting pellet is treated with NH4Cl to lyse red blood cells. The remaining cells are then washed and filtered through a 100 µm nylon mesh. Cell density and viability is assessed. Based on the authors' experience, 6.3×104 to 2.2×105 viable SVF cells can be isolated per mL of aspirated adipose tissue.
The cultured or isolated cells are then seeded on scaffolds including artificial dermis. The artificial dermis should have optimal physical and biochemical factors for its successful use in regenerative medicine. Collagen sponge and hyaluronic acid sheet are two main biomedical materials that are frequently used in clinical medicine. These materials accelerate tissue granulation. Collagen and hyaluronic acid matrices promote migration of host cells and blood vessels into the structure, allowing rapid replacement by host tissue. The cell-scaffold complex is grafted onto the defect area and covered with a polyurethane foam dressing.
Rheinwald and Green et al. (25) succeeded in culturing keratinocytes in 1975. Treatment using cultured autologous keratinocytes was first applied to a burn patient in 1981. Over the past three decades, cultured keratinocytes have been used as autografts or allografts to cover various wounds (26).
Autologous keratinocytes should become an integral part of the management of patients with large burn wounds and patients with limited donor site availability. This treatment can minimize donor-site morbidity when the patient needs a skin graft because it is possible to culture a large sheet of the patient's epidermis from only a small piece of skin. For example, a commercialized product (Holoderm®; Tego Science, Seoul, Korea) is composed of approximately 1 billion keratinocytes. A skin biopsy as small as 1 to 3 cm2 can be expanded 10,000 times in about 14 to 18 days to cover an entire adult body.
Many investigators studied the use of allogeneic keratinocytes in healing partial thickness burns and other wounds (23, 24). They demonstrated that the strategy had impressive therapeutic value.
Previous studies have also shown that cultured allogeneic keratinocytes are effective in the treatment of chronic leg ulcers. Recently, a biological dressing material derived from cultured keratinocytes of a Korean infant's foreskin (Kaloderm®; Tego Science) has newly been developed. A sheet of the cultured allogeneic keratinocytes contains >2×107 cells backed by vaseline gauze. A randomized, controlled, multicenter study has been carried out involving 59 patients with diabetic foot ulcers. Results showed that complete wound healing occurred in all patients (100%) in the keratinocyte-treated group and 69% in the control group (P<0.05) after 12 weeks. The Kaplan-Meier median time to complete ulcer healing was 35 days for the treatment group and 57 days for the control group (Fig. 1) (26).
The application of autologous cultured fibroblasts is one of the representative treatments in the wound coverage. Fibroblasts are the most important mesenchymal cells involved in wound healing (27). The authors have analyzed 16 clinical cases of the tissue-engineered dermis comprising of autologous cultured dermal fibroblasts seeded on a hyaluronic-acid sheet for the coverage of facial defects created by the resection of skin cancer. Based on the authors' experiences, the graft was well taken by all patients. The entire tissue-engineered dermis graft reepithelialized after grafting within 21 to 42 days. All patients had satisfactory results in both functional and cosmetic matters with high quality skin characteristics. Regarding scar contracture, no patient required an additional operation for further improvement of the graft areas. There were no significant complications or recurrences during the follow-up. Patient satisfaction with the tissue-engineered dermis graft was also excellent (Fig. 2).
There is considerable interest in the treatment of chronic wounds with grafts of cultured fibroblasts. Most fibroblast therapy products are composed of cryopreserved allogeneic cells. However, the effect has generally shown to be not very dramatic. One of the possible reasons for the limited effect of these products is that it is difficult for grafted frozen cells to recover, colonize, and persist in chronic wound beds which are deficient in oxygen and nutrients. Moreover, cell activities are impaired due to cryopreservation. Generally, cells in cryopreserved cultures show about 50% viability compared to non-frozen cells, and protein synthesis is inhibited by 70%-98%. In terms of growth factor expression and angiogenesis, there is an even lower rate of recovery (28-30).
To overcome the limitations of such cryopreserved cell allograft techniques, the authors have developed a non-cryopreserved fresh fibroblast allograft method and reported promising results. Based on the authors' clinical study for the treatment of diabetic foot ulcers, the efficacy, safety, and tolerability of fibroblast allograft treatment were excellent. Within 8 weeks, complete wound closure was achieved in 84% of the treatment group and 50% of the control group (P<0.05). There was no adverse effect related to fibroblast allograft treatment (Fig. 3) (28-30).
In recent years, a new sheet comprising of autologous cultured fibroblasts and a scaffold made from the benzyl ester of hyaluronic acid (Hyalograft 3D; ChaBio & Diostec) has been developed in Korea for the treatment of diabetic foot ulcers. Hyaluronic acid is a naturally occurring biopolymer whose molecular structure is highly conserved between mammalian species. First described in 1934, it has since been used across a wide variety of medical fields as diverse as neurosurgery and cutaneous wound healing. The benzyl ester of hyaluronic acid is an ideal material for wound healing, as it is biocompatible and resorbable and integrates with ulcer tissues. Based on the authors' clinical trial study with 12 week follow-up, complete wound healing occurred in 84% in the autologous fibroblast-hyaluronic acid complex-treated group and in 34% in the control group (P<0.01). The safety and tolerability were also excellent.
As mentioned earlier, utilizing cultured cells for clinical purposes requires FDA-approved facilities and techniques and a lengthy culture period. Adipose-derived SVF cells can substitute for fibroblasts. The authors have fairly extensive clinical cases of the tissue-engineered dermis using the SVF cells for the coverage of defects on the face, hand, and foot. Based on the authors' experience, the results were favorable (Fig. 4 and 5) (31-35).
The SVF cells can be also applied for chronic wounds. An in vitro study has been performed to determine the effect of cell therapy using uncultured SVF cells on cell proliferation and collagen synthesis of diabetic fibroblasts, which are the major contributing factors in wound healing (36). The results showed that cell proliferation and collagen synthesis in the SVF cell treatment group were 28% and 44% higher than those in the control group, respectively. In addition, a synergic effect of the SVF cell autograft was observed possibly due to mutual stimulation of the diabetic fibroblasts and SVF cells by growth factors secreted from them. A clinical trial study, which included 54 patients with diabetic foot ulcers, also demonstrated that SVF cell therapy accelerate diabetic wound healing. After 8 weeks of treatments, complete wound healing occurred in all patients (100%) in the SVF cell treated group and in 16 patients (62%) in the control group (P<0.05) (36).
Bone marrow stroma is the source of mesenchymal stem cells which may serve as long-lasting precursors for bones, cartilages, muscles, and connective tissues. Mesenchymal stem cells also have a low immunity-assisted rejection rate, and they have a greater ability to divide without apoptosis than differentiated cells. Therefore, they have been drawing intense attention in the field of bioengineering.
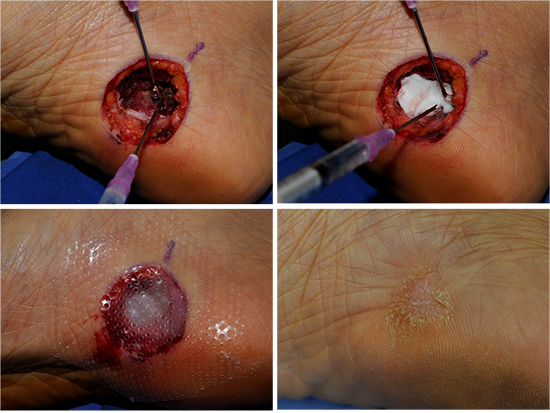
At present, however, it is difficult for a clinician to utilize the cultured bone marrow stromal stem cells (BSCs) for the purpose of wound healing due to issues regarding the approval by the FDA. The authors have used uncultured BSCs in selected cases with exposed bone on the wound bed, especially on the tibial area. The BSCs in the tibia can be taken out by drilling in the bone and then applied on the wound bed of the exposed bony area. The results were favorable (Fig. 6).
Attempts to deliver growth factors to wounds utilizing platelets have been developed and have shown beneficial efficacy on chronic wound healing (32, 37). The usefulness of autologous platelets in chronic wound healing has been demonstrated by many studies. Moreover, some authors have also tried homologous platelets to treat chronic wounds, and have concluded that homologous platelets are as effective as autologous equivalents. However, in the case of the autologous platelets, patient blood has to be aspirated and processed to yield the required platelet products. Moreover, patients with chronic wounds usually have poor general health and anemia, and thus the large volume blood withdrawal required to produce autologous platelet-rich plasma or platelet gel may have detrimental effects on hemodynamic stability. In addition, equipment and techniques are used to separate platelets, and although homologous platelet gels have been used, healthy donors are required for its production, and testing of blood samples must be done for a history of infectious diseases.
To overcome these limitations, the authors performed serial studies to determine the potential of a straightforward method using a blood bank platelet concentrate (BBPC). BBPC is a transfusable blood product that is used to restore low platelet counts. A BBPC is readily available and 1 unit contains approximately 5×1010 platelets in 50 mL of plasma. The results demonstrated that the use of a BBPC can provide a simple, safe, and effective means of treating diabetic foot ulcers (Fig. 7) (38, 39).
Keratinocytes mainly release growth factors (TGF-α, PDGF, bFGF, VEGF, and TGF-β) and cytokines (IL-1, -6, -8, and -10), but lack the ability to secrete extracellular matrices. Fibroblasts transplanted onto the wound secrete various cytokines and growth factors that control cell proliferation, induce angiogenesis, and modify the inflammatory process. They also produce three-dimensional extracellular matrices comprising collagens, proteoglycans, and other proteins. Therefore, the overall environment of wounds can be improved by controlling cell proliferation, inducing angiogenesis, and stimulating production of the three-dimensional extracellular matrices.
SVF cells can be obtained large quantities without cell culture. They contain heterogenous cell populations, including fibroblasts, stem cells, endothelial cells, mast cells, pericytes, preadipocytes, smooth muscle cells, and progenitor cells, which are well-known to accelerate wound healing. Based on the results of Suga et al. (40), SVF cells contain blood-derived cells (37%), adipose-derived stem/stromal cells (37%), endothelial cells (15%), and other cells (11%). All these SVF cells grafted on the wound bed could not only stimulate host cells around the wound, but also be taken on the wound bed and function independently by providing growth factors and extracellular matrices due to the autologous nature of the cells.
Bone marrow stromal stem cells (BSCs) are superior to fibroblasts in wound healing activities. BSCs produce collagen and growth factors much higher than fibroblasts. In particular, the VEGF synthesis of the BSCs is 12 times higher than that of fibroblasts. These results suggest that BSCs may possibly be used as a replacement for fibroblasts, which are commonly being used currently for wound healing (41-44).
Platelets contain at least seven locally acting growth factors from alpha-granules, i.e., three isomers of platelet derived growth factor (PDGF-AA, PDGF-AB, and PDGF-BB), two isomers of transforming growth factor-β (TGF-β1 and TGF-β2), vascular endothelial growth factor (VEGF), and epidermal growth factor (EGF). Platelets applied on chronic wounds secrete various cytokines and growth factors, which are absent in chronic wounds. They can compensate for impaired activities of cells crucial for wound healing.
As the populations of industrialized countries age and become more sedentary and increasing number of patients seek a procedure of the least degree of invasiveness in wound repair, cell therapy for wound healing should be increasing dramatically. To meet these demands, various commercially available cell-scaffold complexes have been developed and will be widely used. More and more, cell therapy methods are replacing the conventional tissue transfer.
Stem cells hold great promise for addressing the need for viable cell sources because they share the advantages of both allogeneic and autologous cells. Mesenchymal stem cells (MSCs) have the ability to proliferate, form extensive colonies of healthy newly differentiated fibroblasts, demonstrate low levels of immunity-assisted rejection, and divide without apoptosis than differentiated cells. In addition, it was demonstrated that even after 20 or 30 cycles of cell doubling in culture, they still retain stem cell properties. Accordingly, MSCs have attracted much attention in the bioengineering field. In the meantime, bone marrow stromal MSCs and umbilical cord blood-derived MSCs are on the way of research for this purpose.
While a lot of reports regarding cell therapy for wound healing have been published, clinically available treatments are still limited. There appears to be sufficient evidence to support the use of cell therapy to facilitate wound healing in chronic wounds. However, clinical research regarding the cell therapy for acute wounds is limited. Further controlled studies are required before this modality is considered a standard treatment for healing of acute wounds.
There is limited information of "take" rates of the grafted cells. Inconsistent "take" rates have been reported. These reports attribute their engraftment failure to the sensitivity of the cultured cells to bacterial colonization and inappropriate wound beds.
In applying cell therapy for healing of chronic wounds, the most important point is to identify good responders to cell therapy. Before cells are applied, the wound condition should be assessed for appropriateness. The wound bed should be healthy and clean with or without granulation tissue. There should not be any accompanying devitalized tissue, increased drainage, increase in size or odor. Wound edges should be open to allow for epithelial migration. Periwound tissue should not be macerated or indurated. It is important to emphasize that these treatment modalities must be used along with other standard principles of chronic wound management, including debridement, infection control, pressure off-loading, and revascularization. Without adhering to these important principles, addition of an active adjunctive modality is unlikely to result in improved healing in chronic wound patients. Lastly, it must be emphasized that non-commercialized cell therapies need to be approved by the FDA.
Future research should be focused on improving wound bed preparation and infection control to maximize cell engraftment, expediting cell culturing, and looking at long-term patient follow-ups both functional and aesthetic. Accurate identification of the causes and the appropriate cell therapy methods are therefore mandatory to obtain the best treatment outcomes.
To establish cell therapy as a standard treatment, more investigation with a larger number of patients is necessary. In addition, further studies are needed to determine the fate of transplanted cells and the number of cells required to show definitive effects. Further studies on the length of time for cells to be maintained after harvesting and still retain viability are also required.
Figures and Tables
Fig. 1
Treatment of a diabetic foot ulcer using an allogeneic keratinocyte sheet. A woman (aged 63 yr) with a chronic non-healing diabetic ulcer over 10 weeks old on the medial side of the right fifth toe. After debridement, freshly bleeding wound bed was prepared. (A) View of the cultured allogeneic keratinocyte sheet. (B) Preoperative view. (C-E) Wound appearance was taken every week. Re-epithelization occurred progressively from the periphery to the center of the wound. The wound was completely healed after 3 weeks.

Fig. 2
Cheek defect created by removal of a basal cell carcinoma was reconstructed by autologous fibroblasts seeded on a hyaluronic acid sheet. The entire wound re-epithelialized 21 days after grafting. (A and B) Fibroblast pellet and non-woven hyaluronic acid sheet. (C) After wide excision of the tumor. (D) Immediately after the graft of the tissue-engineered dermis. (E) One-year after the graft. The result demonstrates excellent color match with minimal scar contracture.

Fig. 3
A woman (aged 50 yr) with 6 week old chronic non-healing diabetic ulcers on the left second and third toe tips. The second toe with the larger wound was treated with the fibroblast allograft, and the third toe with the smaller wound was conservatively treated. (A) Preoperative view. (B) Immediate after treatments. (C) Two weeks after treatments. (D) Twenty days after treatments, the second toe wound completely epithelized, but the third toe wound (control) had a raw surface. (E) Three months after treatments, the second toe was good with a healthy texture, but the third toe had not yet completely epithelized.
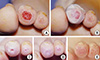
Fig. 4
Reconstruction using stromal vascular fraction (SVF) cells. (A) A squamous cell carcinoma on the hand. (B) After wide excision of the tumor, flap surgery was required since the first metacarpal bone and tendons were exposed. However, a procedure which needs a general anesthesia or a long operation time could not be performed due to the poor general health condition of the patient. (C and D) To prepare the wound bed to be allowed for a skin graft, stromal vascular cells were isolated and grafted on the wound bed. (E) Three weeks after the graft, a healthy granulation tissue was formed. (F) The wound was finally closed by a split-thickness skin graft under local anesthesia.
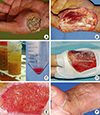
Fig. 5
Results of a stromal vascular fraction (SVF) cell graft applied to a thumb. (A) A bone and pulp defect on the thumb caused by trauma. Black and yellow arrows indicate the cortex and medulla of the distal phalanx, respectively. (B) SVF cells suspended in fibrin glue were transplanted one day after trauma. (C-F) Two-weeks, 4-weeks, 3-months, and 1-yr after the graft. The wound was completely closed 4 weeks after the graft.
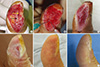
Fig. 6
A 73-yr-old man with diabetes mellitus had a non-healing ulcer over 8 weeks old on the anterior tibial area of the lower leg. (A) A large wound on the lower leg with exposed tibial bone (an arrow). (B) Drilling in the bone to take out bone marrow stromal stem cells (BSCs). (C-E) Two, 3, and 4 weeks after drilling. Granulation tissue was formed from the drilling sites. (F) The wound was closed by a skin graft.

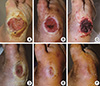
Fig. 7
A 67-yr-old man with diabetes mellitus had a non-healing ulcer on the lateral border of his right foot at the metatarsophalangeal joint level. Before participating in the study, the patient had been treated for 12 weeks. (A) Preoperative view. (B) After surgical debridement, the metatarsal and proximal phalangeal bones were exposed. (C) Three days after the first application of blood bank platelet concentrate (BBPC) (immediate before the second application of the BBPC). (D) Three weeks after the first application of BBPC. (E) Five weeks after the first application of BBPC. (F) Seven weeks after treatment, the wound was completely epithelialized. Two applications were performed for this patient.
References
1. Moon HS, Burm JS, Yang WY, Kang SY. Prognosis of full-thickness skin defects in premature infants. Arch Plast Surg. 2012; 39:463–468.
2. Park YS, Lee JW, Huh GY, Koh JH, Seo DK, Choi JK, Jang YC. Algorithm for primary full-thickness skin grafting in pediatric hand burns. Arch Plast Surg. 2012; 39:483–488.
3. Yun MJ, Park JU, Kwon ST. Surgical options for malignant skin tumors of the hand. Arch Plast Surg. 2013; 40:238–243.
4. Bae SH, Bae YC, Nam SB, Choi SJ. A skin fixation method for decreasing the influence of wound contraction on wound healing in a rat model. Arch Plast Surg. 2012; 39:457–462.
5. Han SK, Yoon WY, Jeong SH, Kim WK. Facial dermis grafts after removal of basal cell carcinomas. J Craniofac Surg. 2012; 23:1895–1897.
6. Han SK, You HJ. Wound coverage using advanced technology in Korea. J Korean Med Assoc. 2011; 54:594–603.
7. Gu JH, Han SK, Jeong SH, Kim WK. Hand coverage using venous island flaps. J Plast Reconstr Aesthet Surg. 2012; 65:e366–e367.
8. Han SK, Lee BI, Kim WK. The reverse digital artery island flap: an update. Plast Reconstr Surg. 2004; 113:1753–1755.
9. Han SK, Lee BI, Kim WK. The reverse digital artery island flap: clinical experience in 120 fingers. Plast Reconstr Surg. 1998; 101:1006–1011.
10. Ghanem AM, Hachach-Haram N, Leung CC, Myers SR. A systematic review of evidence for education and training interventions in microsurgery. Arch Plast Surg. 2013; 40:312–319.
11. Myers SR, Froschauer S, Akelina Y, Tos P, Kim JT, Ghanem AM. Microsurgery training for the twenty-first century. Arch Plast Surg. 2013; 40:302–303.
12. Whang KK, Kim MJ, Song WK, Cho S. Comparative treatment of giant congenital melanocytic nevi with curettage or Er: YAG laser ablation alone versus with cultured epithelial autografts. Dermatol Surg. 2005; 31:1660–1667.
13. Sood R, Roggy D, Zieger M, Balledux J, Chaudhari S, Koumanis DJ, Mir HS, Cohen A, Knipe C, Gabehart K, et al. Cultured epithelial autografts for coverage of large burn wounds in eighty-eight patients: the Indiana University experience. J Burn Care Res. 2010; 31:559–568.
14. Kim HR, Han SK, Rha SW, Kim HS, Kim WK. Effect of percutaneous transluminal angioplasty on tissue oxygenation in ischemic diabetic feet. Wound Repair Regen. 2011; 19:19–24.
15. Park DJ, Han SK, Kim WK. Is the foot elevation the optimal position for wound healing of a diabetic foot? J Plast Reconstr Aesthet Surg. 2010; 63:561–564.
16. Seo YK, Song KY, Kim YJ, Park JK. Wound healing effect of acellular artificial dermis containing extracellular matrix secreted by human skin fibroblasts. Artif Organs. 2007; 31:509–520.
17. Erdag G, Sheridan RL. Fibroblasts improve performance of cultured composite skin substitutes on athymic mice. Burns. 2004; 30:322–328.
18. Morimoto N, Saso Y, Tomihata K, Taira T, Takahashi Y, Ohta M, Suzuki S. Viability and function of autologous and allogeneic fibroblasts seeded in dermal substitutes after implantation. J Surg Res. 2005; 125:56–67.
19. Yates CC, Whaley D, Wells A. Transplanted fibroblasts prevents dysfunctional repair in a murine CXCR3-deficient scarring model. Cell Transplant. 2012; 21:919–931.
20. El-Ghalbzouri A, Gibbs S, Lamme E, Van Blitterswijk CA, Ponec M. Effect of fibroblasts on epidermal regeneration. Br J Dermatol. 2002; 147:230–243.
21. Kang BS, Na YC, Jin YW. Comparison of the wound healing effect of cellulose and gelatin: an in vivo study. Arch Plast Surg. 2012; 39:317–321.
22. Kim H, Son D, Choi TH, Jung S, Kwon S, Kim J, Han K. Evaluation of an amniotic membrane-collagen dermal substitute in the management of full-thickness skin defects in a pig. Arch Plast Surg. 2013; 40:11–18.
23. Gallego L, Junquera L, Villarreal P, Peña I, Meana A. Use of cultured human epithelium for coverage: a defect of radial forearm free flap donor site. Med Oral Patol Oral Cir Bucal. 2010; 15:e58–e60.
24. Yanaga H, Udoh Y, Yamauchi T, Yamamoto M, Kiyokawa K, Inoue Y, Tai Y. Cryopreserved cultured epidermal allografts achieved early closure of wounds and reduced scar formation in deep partial-thickness burn wounds (DDB) and split-thickness skin donor sites of pediatric patients. Burns. 2001; 27:689–698.
25. Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975; 6:331–343.
26. You HJ, Han SK, Lee JW, Chang H. Treatment of diabetic foot ulcers using cultured allogeneic keratinocytes: a pilot study. Wound Repair Regen. 2012; 20:491–499.
27. Zou SB, Yoon WY, Han SK, Jeong SH, Cui ZJ, Kim WK. Cytotoxicity of silver dressings on diabetic fibroblasts. Int Wound J. 2013; 10:306–312.
28. Han SK, Choi KJ, Kim WK. Clinical application of fresh fibroblast allografts for the treatment of diabetic foot ulcers: a pilot study. Plast Reconstr Surg. 2004; 114:1783–1789.
29. Han SK, Kim WK. Revisiting fresh fibroblast allograft as a treatment for diabetic foot ulcers. Plast Reconstr Surg. 2009; 123:88e–89e.
30. Han SK, Kim HS, Kim WK. Efficacy and safety of fresh fibroblast allografts in the treatment of diabetic foot ulcers. Dermatol Surg. 2009; 35:1342–1348.
31. Castro-Govea Y, De La Garza-Pineda O, Lara-Arias J, Chacón-Martínez H, Mecott-Rivera G, Salazar-Lozano A, Valdes-Flores E. Cell-assisted lipotransfer for the treatment of parry-romberg syndrome. Arch Plast Surg. 2012; 39:659–662.
32. Choi J, Minn KW, Chang H. The efficacy and safety of platelet-rich plasma and adipose-derived stem cells: an update. Arch Plast Surg. 2012; 39:585–592.
33. Lee JH, Lee KH, Kim MH, Kim JP, Lee SJ, Yoon J. Possibility of undifferentiated human thigh adipose stem cells differentiating into functional hepatocytes. Arch Plast Surg. 2012; 39:593–599.
34. Lee SK, Kim DW, Dhong ES, Park SH, Yoon ES. Facial soft tissue augmentation using autologous fat mixed with stromal vascular fraction. Arch Plast Surg. 2012; 39:534–539.
35. Sung HM, Suh IS, Lee HB, Tak KS, Moon KM, Jung MS. Case reports of adipose-derived stem cell therapy for nasal skin necrosis after filler injection. Arch Plast Surg. 2012; 39:51–54.
36. Han SK, Kim HR, Kim WK. The treatment of diabetic foot ulcers with uncultured, processed lipoaspirate cells: a pilot study. Wound Repair Regen. 2010; 18:342–348.
37. Shin HS, Oh HY. The effect of platelet-rich plasma on wounds of OLETF rats using expression of matrix metalloproteinase-2 and -9 mRNA. Arch Plast Surg. 2012; 39:106–112.
38. Jeong SH, Han SK, Kim WK. Treatment of diabetic foot ulcers using a blood bank platelet concentrate. Plast Reconstr Surg. 2010; 125:944–952.
39. Han SK, Kim DW, Jeong SH, Hong YT, Woo HS, Kim WK, Gottrup F. Potential use of blood bank platelet concentrates to accelerate wound healing of diabetic ulcers. Ann Plast Surg. 2007; 59:532–537.
40. Suga H, Matsumoto D, Inoue K, Shigeura T, Eto H, Aoi N, Kato H, Abe H, Yoshimura K. Numerical measurement of viable and nonviable adipocytes and other cellular components in aspirated fat tissue. Plast Reconstr Surg. 2008; 122:103–114.
41. Kim JB, Chun KW, Han SK, Kim WK. Effect of human bone marrow stromal cell allograft on proliferation and collagen synthesis of diabetic fibroblasts in vitro. J Plast Reconstr Aesthet Surg. 2010; 63:1030–1035.
42. Lee CH, Han SK, Choi WI, Kim WK. Effect of human bone marrow stromal cells and dermal fibroblasts on collagen synthesis and epithelization. Ann Plast Surg. 2007; 59:713–719.
43. Han SK, Chun KW, Gye MS, Kim WK. The effect of human bone marrow stromal cells and dermal fibroblasts on angiogenesis. Plast Reconstr Surg. 2006; 117:829–835.
44. Han SK, Yoon TH, Lee DG, Lee MA, Kim WK. Potential of human bone marrow stromal cells to accelerate wound healing in vitro. Ann Plast Surg. 2005; 55:414–419.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download