Abstract
A 78-yr-old woman presented with gross hematuria for 2 weeks. On cystoscopy, a frond-like mass was observed at the bladder trigone. Transurethral resection of bladder tumor was performed for the mass. Histopathological findings showed that 90% of lesions were lymphoepithelioma-like carcinoma (LELCA) and a few lesions were non-invasive transitional cell carcinoma. On microscopy, syncytial growth pattern and indistinct cytoplasmic borders were observed with the severe infiltration of lymphoid cells. The case was followed-up for 8 months without recurrence. This is the first report of a LELCA case in Korea.
Lymphoepithelioma is a term used to designate an undifferentiated malignant epithelial tumor of the nasopharynx that is histologically distinctive because of a markedly prominent lymphoid infiltrate (1).
Carcinomas with similar histological features arising outside the nasopharynx are called lymphoepithelioma-like carcinoma (LELCA). LELCA occurs in organs such as salivary glands, the uterine cervix, the thymus, the lung, the skin, the stomach, the bladder, the prostate, and the breast (2).
It is very rare that LELCA occurs in the urinary system, there is one case where it occurred in the renal pelvis, and one case where it occurred in the ureter and the kidney simultaneously, but has not been reported so far in urinary bladder in Korea. The author et al. have lately experienced LELCA that occurred in the urinary bladder, and thus have reported the case along with philological considerations.
A 78-yr-old woman presented with gross hematuria for 2 weeks on November 23, 2009. There was no history of urologic problems. The patient was a non-smoker and had not been exposed to carcinogen. Physical examination and vital sign were normal
At the time when the patient was admitted to this hospital, numerous RBC were observed on urinalysis. There were no notable findings on blood test and chest radiography. On cystoscopy, a frond-like mass was observed at the bladder trigone, which measured about 1 cm (Fig. 1). Since hematuria continued, Foley catheterization was performed.
On computerized tomography (CT) of the abdomen and pelvis, the findings of enhancement and a small-sized mass were observed on the inner surface between posterior walls in the bladder trigone. The findings of perivesical infiltration and lymph node metastasis were not observed.
On the authority of above findings, the authors suspected the case to be carcinoma in situ of the urinary bladder or transitional cell carcinoma, and thus transurethral resection of bladder tumor (TURBT) was performed.
On histopathological examination, it was found that 90% of lesions were LELCA and a few lesions were non-invasive transitional cell carcinoma.
On microscopy, syncytial growth pattern and indistinct cytoplasmic borders were observed with the severe infiltration of lymphoid cells. The findings of necrosis and mitosis were observed in many regions. It was found that tumor cells invaded the whole lamina propria. Non-invasive transitional cell carcinoma was concomitant fractionally (Fig. 2).
On immunohistochemical staining, tumor cells were positive for cytokeratin 7 but were negative for cytokeratin 20 (Fig. 3). The infiltrated lymphocytes were composed of abundant CD3 positive T cells and CD20 positive B cells. For leukocyte common antigen (LCA), lymphoid cells were positive but tumor cells were negative (Fig. 4). In situ hybridization for Epstein-Barr virus was negative.
The patient has been without recurrence and metastasis for 3 months after the operation, and currently is on a follow-up.
LELCA of the urinary bladder is rare. LELCA of the urinary bladder, first reported by Zuckerberg et al. (3) in 1991, is uncommon with a reported incidence between 0.4 and 1.3% of all bladder carcinoma (4).
In the urinary tract, they typically arise in the urinary bladder, although isolated cases have been reported in the renal pelvis, ureter, and urethra (5, 6).
As suggested by Amin et al. (7), LELCA was categorized as pure (100%), predominantly (more than 50%), or focal (less than 50%).
If other classification is applied, LELCA was classified as pure when 100% of the tumor showed lymphoepithelioma-like carcinoma pattern, and mixed when associated with usual infiltrating urothelial carcinoma, adenocarcinoma, or squamous carcinoma (2).
It has been suggested that pure/predominant LELCA responds to chemotherapy and may best be treated with bladder preservation therapy (1, 13). According to previous reports, pure and predominant LELCA is more favorable than focal LELCA in prognosis (2, 3, 7).
This case was the predominant type that LELCA accounted for over 90% of lesions and was fractionally accompanied with non-invasive transitional cell carcinoma. The differential diagnosis is usually malignant lymphoma, invasive transitional cell carcinoma, squamous cell carcinoma and small cell carcinoma. It is particularly important to distinguish from malignant lymphoma. Amin et al. (5) note that it is imperative to distinguish between LELCA and malignant lymphoma, as primary bladder lymphoma is extremely rare. Therefore, immunochemical staining, such as LCA and keratin, may be used for differentiation.
Siegelbaum et al. (8) maintained that immunochemical staining techniques were helpful to distinguish bladder lymphoma from undifferentiated carcinoma.
Cystoscopic finding or CT finding is not helpful to distinguish LELCA from bladder carcinoma. It is possible to differentiate bladder carcinoma from LELCA by microscopic finding. Pooly differentiated transitional cell carcinoma (TCC) with a lymphoid linfiltrate should be distinguishable from LELCA in that the latter is characterizied by syncytia of tumor cell, vesicular nuclei and prominent nuclei (7).
The microscopic findings of LELCA are characterized by the indistinct cytoplasmic border and the syncytial growth pattern with the prominent lymphocytic infiltrate. It is important to check cytokeratin in tumor cells on immunochemical staining, in order to ascertain that cells are originated from epithelial cells (1).
The principal symptoms of LELCA were mostly gross hematuria, solitary mass, and tumors measured 1 to 5 cm (10, 12). The Epstein-Barr virus is regarded as one of factors of the LELCA that occurred in the thymus gland (9). However, it has not been elucidated that LELCA of the urinary system is related with EBV (10), Gulley et al. (11) reported that EBV, detected from nasophayneal carcinoma, was not detected in 9 out of 11 cases of LELCA. Also in 9 cases of LELCA reported by Holmang et al. (12), EBV was not detected. Likewise, EBV was not detected in this case. For LELCA treatment, surgical therapy and chemotherapy can be applied.
In the case of small tumors that measure 5 cm and less, TURBT is applied, but in the case of big or invasive tumors, radical cystectomy may be applied. As nasopharyngeal lymphoepithelioma is well reacted to chemotherapy, methotrexate, vinblastine, doxorubicin and cisplatin may be applied to chemotherapy (7). Dinney et al. (13) reported that primary chemotherapy was performed on 3 patients with muscle invasive lymphoepithelioma of the bladder and as a result their bladder functions were salvaged.
It is presumed that bladder lymphoepithelioma may be well reacted to radiotherapy, like nasopharyngeal lymphoepithelioma (14). Holmang et al. (12) applied radiotherapy to 4 patients, but could not evaluate whether the patients got cured successfully. However, the guideline on LELCA treatment has not been established yet.
LELCA is a rare tumor, and it is important to differentiate it from other tumors. In addition, it is necessary to establish the definitive standard of its treatment.
Figures and Tables
Fig. 2
Mircoscopic finding. (A) Lymphoepithelioma-like carcinoma, syncytial pattern with prominent lymphocytic infiltrate. (B) Non-invasive transitional cell carcinoma with lymphoepithelioma-like carcinoma.
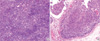
Fig. 3
Cytokerain staining. (A) Immunochemical staining with antibodies against cytokeratin 7 is positive. (B) Negative immunochemical staining with antibodies against cytokeratin 20.

Fig. 4
Immunochemical staining. (A) Positive staining with antibodies against CD3 shows abundant T-cells. (B) Positive staining with antibodies against CD20 shows abundant B-cells. (C) Leukocyte common antigen (LCA) stains lymphocytes (left). Non-invasive transitional cell carcinoma is negative for LCA (right).
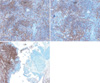
References
1. Carbone A, Micheau C. Pitfalls in the microscopic diagnosis of undifferentiated carcinoma of nasopharyngeal type (lymphoephithelioma). Cancer. 1982. 50:1344–1351.
2. Tamas EF, Nielsen ME, Schoenberg MP, Epstein JI. Lymphoepithelioma-like carcinoma of the urinary tract: a clinicopathological study of 30 pure and mixed cases. Mod Pathol. 2007. 20:828–834.

3. Zukerberg LR, Harris NL, Young RH. Carcinoma of the urinary bladder simulating malignant lymphoma: A report of five cases. Am J Surg Pathol. 1991. 15:569–576.
4. Porcaro AB, Gilioli E, Migliorini F, Antoniolli SZ, Iannucci A, Comunale L. Primary lymphoepithelioma-like carcinoma of the urinary bladder: report of one case with review and update of the literature after a pooled analysis of 43 patients. Int Urol Nephrol. 2003. 35:99–106.
5. Cohen RJ, Stanley JC, Dawkins HJ. Lymphoepithelioma-like carcinoma of the renal pelvis. Pathology. 1999. 31:434–435.

6. Izquierdo-García FM, García-Díez F, Fernández I, Pérez-Rosado A, Sáez A, Suárez-Vilela D, Guerreiro-González R, Benéitez-Alvarez M. Lymphoepithelioma-like carcinoma of the bladder: three cases with clinicopathological and p53 protein expression study. Virchows Arch. 2004. 444:420–425.
7. Amin MB, Ro JY, Lee KM, Ordóñez NG, Dinney CP, Gulley ML, Ayala AG. Lymphoepithelioma-like carcinoma of the urinary bladder. Am J Surg Pathol. 1994. 18:466–473.

8. Siegelbaum MH, Edmonds P, Seidmon EJ. Use of immunohistochemistry for identification of primary lymphoma of the bladder. J Urol. 1986. 136:1074–1076.

9. Ward JN, Dong WF, Pitts WR Jr. Lymphoepithelioma-like carcinoma of the bladder. J Urol. 2002. 167:2523–2524.

10. Kang DK, Chung HS, Park CM, Kim HK, Park JY, Kang GH, Ro JY. Multiple oragn involvement of lymphoepithelioma-like carcinoma in the kidney and the ureter. Korean J Urol. 2003. 44:297–299.
11. Gulley ML, Amin MB, Nicholls JM, Banks PM, Ayala AG, Srigley JR, Eagan PA, Ro JY. Epstein-Barr virus is detected in undifferentiated nasopharyngeal carcinoma but not in lymphoepithelioma-like carcinoma of the urinary bladder. Hum Pathol. 1995. 26:1207–1214.

12. Holmäng S, Borghede G, Johansson SL. Bladder carcinoma with lymphoepithelioma-like differentiation: a report of 9 cases. J Urol. 1998. 159:779–782.
13. Dinney CP, Ro JY, Babaian RJ, Johnson DE. Lymphoepithelioma of the bladder: a clinicopathological study of 3 cases. J Urol. 1993. 149:840–841.

14. Kuten A, Cohen Y, Lavie R, Dale J, Ben Arush MB, Goldberg H, Stein M. Update on nasopharyngeal carcinoma in northern Israel. Strahlenther Onkol. 1994. 170:565–570.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download