Abstract
Purpose
Hepatocyte growth factor (HGF) and its receptor (HGFR/c-Met) regulate motility, mitogenesis, and morphogenesis in a cell type-dependent fashion. We report the role of HGF and c-Met on stress-induced ARPE-19 human retinal pigment epithelial (RPE) cells in this study.
Methods
The cells were cultured either with or without serum. Southern and Western blot analyses were done to determine the expression patterns of HGF/c-Met in serum-starved ARPE-19 cells. The cell proliferation pattern in serum-starved condition was analyzed using MTS assay. Inhibition level of cell proliferation was analyzed using a neutralizing monoclonal antibody against c-Met (2 µg/ml).
Results
Abnormal cell proliferation and scattering of ARPE-19 cells was observed under serum starvation. HGF/c-Met were expressed in serum-starved ARPE-19 cells. ARPE-19 cell proliferation was also enhanced with recombinant HGF treatment. Neutralization against c-Met inhibited the proliferation of serum-deprived ARPE-19 by 64.5% (n=9, S.D. 5.5%). Serum starvation appears to induce epithelial-mesenchymal transition of ARPE-19 cells, resulting in scatter, and the expression of α-smooth muscle actin (α-SMA), a marker for fibrosis.
In normal healthy eyes, retinal pigment epithelial (RPE) cells form a polarized monolayer adjacent to the photoreceptors and are involved in various activities that are essential to retinal homeostasis and visual function. RPE cells are commonly activated by failure of retinal adhesion that causes proliferative vitreoretinopathy (PVR). Scar tissue develops as epiretinal and subretinal membranes, and this causes a pathological condition in which these cells become activated and mobilized.1 RPE cells are a key component of epiretinal and subretinal membranes, and in those eyes that develop PVR, the RPE cells beneath the detached neural retina undergo an epithelial-to-mesenchymal transition that involves a wide range of changes. The epithelium loses its hexagonal shape and resembles fibroblasts. The cells undergo a change from being a static non-dividing cell becoming a migratory and proliferating variant.2 Transition of RPE cells is modulated by soluble growth factors and cytokines such as tumor necrosis factor-α (TNF-α), interlukin-1 (IL-1) and platelet-derived growth factor (PDGF).3 The basic pathomolecular mechanism by which the sedentary RPE cells become activated is still poorly understood. Among the involved factors, hepatocyte growth factor (HGF) or scatter factor (SF) is associated with increasing the mobility of various types of epithelium.2,4
HGF was originally identified as a potent mitogen for hepatocytes and it was purified as a disulfide-linked heterodimer consisting of a 62-kDa subunit and a 37-kDa subunit. Pro-HGF is subsequently cleaved by one of several proteases (e.g. uPA and tPA) between the sites Arg494 and Val495 to form the active, heterodimeric molecule that has a high affinity for its receptor, c-Met.5-7 HGF/c-Met signaling has been shown to trigger a variety of cellular responses that can vary based upon the cellular context. In vitro studies have revealed that activation of c-Met by HGF/SF leads to proliferation of hepatocytes,8 renal tubule cells and endothelial cells,9 stimulation of cell dissociation and mobility,10 i.e. scattering, and stimulation of cell movement through the extracellular matrix, i.e. invasion.11,12 Furthermore, signaling via this pathway has been shown to induce certain epithelial and mesenchymal cell types to grow into a three-dimensional matrix undergoing a branching morphogenesis.13-15 This is a differentiation program in which groups of cells proliferate, migrate and differentiate in such a way as to form a connected series of tubules arranged like the branches of a tree.16 HGF also possesses mitogenic, motogenic and morphogenic properties and it has recently been implicated in various retinal diseases.7,17-21
The present study was undertaken to determine if RPE cells are activated for the maintenance of homeostasis after serum starvation, a pathological condition, and if their activation involves HGF/c-Met.
The Dig labeling kit, digoxigenin-alkaline phosphatase Fab fragments and CSPD-star ready-to-use were acquired from Roche Molecular Biochemicals (Indianapolis, IN, USA). The primers were custom synthesized by Jenotec (Daejoen, Korea). Rabbit anti-human c-Met polyclonal antibody and monoclonal antibody to human c-Met were acquired from Santa Cruz Biotechnology (C-28, Santa Cruz, CA, USA) and R&D systems (Minneapolis, MN, USA). Goat anti-human HGF polyclonal antibody (H-20) and horseradish peroxidase conjugated goat anti-mouse, anti-rabbit and anti-sheep IgG was supplied by Santa Crus Biotechnology (Santa Cruz, CA, USA). Nupage gels were acquired from Invitrogen (Carlsbad, CA, USA). Minimal essential media (MEM), fetal bovine serum (FBS), gentamycin were purchased from Life Technology/Gibco BRL (Grand Island, NY, USA).
Cultured primary cells are not suitable for evaluation of cell growth because HGF/c-Met is expressed,22 so cellular growth of human RPE cell line (ARPE-19 from ATCC) cells was determined in a serum-free condition. ARPE-19 cells were grown in Dulbecco's modified Eagle's medium/nutrient F12 (DMEM-F12) supplemented with 10% fetal bovine serum and 25 µg/ml gentamycin. Cells (2×104) were seeded in 60-mm dishes containing 5% serum-containing medium and incubated for 16 hours. The serum supply was then halted and cells were cultured in serum-free medium for 0, 1, 3, 5, or 7 days. Finally, the cells were harvested by trypsinization, washed with PBS, and stored at minus 70℃. The supernatant medium obtained from each day was concentrated with a Centricon unit (Millipore, MA, USA), and also stored at -70℃ for Western blot analysis.
Serum starved ARPE-19 cells were incubated for 24 hours with or without monoclonal mouse anti-human c-Met antibody. After incubation, the cells were lysed by washingtwice with ice cold PBS, and then resuspended with RIPA buffer [1 % Triton X-100, 5% SDS, 5% Deoxycholic acid, 0.5 M Tris-Cl (pH 7.5), 10 % glycerol, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride (PMSF), 5 µg/µl aprotinin, 1 µg/µl leupeptin, 1 µg/µl pepstatin, 200 mM sodium orthovandates and 200 mM sodium fluoride]. Cell lysates were incubated on ice for 10 minutes, then centrifuged at 10,000 g for 25 minutes at 4℃. The total protein of the cell lysates and concentrated medium was measured using a standard BCA assay (Pierce, Rockford, USA). Fifteen µg of cell lysates were resuspended at a 4:1 ratio into 5 x sample buffer [60 mM Tris-HCl (pH 7.4), 25% glycerol, 2% SDS, 14.4 mM 2-mercaptoethanol, 0.1% bromophenol blue], boiled for 5 minutes and then resolved with SDS-PAGE.
The proteins were transferred to a nitrocellulose membrane (Hybond-C, Amersham Phamarcia Biotech, Germany), and then stained with Ponseau S (Sigma, USA) to visualize protein bands and ensure equal protein loading. The membrane was blocked for 45 minutes with 5% non-fat skim milk in TBS buffer [20 mM Tris-HCl pH 7.6, 137 mM NaCl, 0.1% Tween-20]. The blots were incubated for an hour using 0.2 µg/ml rabbit anti human c-Met polyclonal antibody and goat anti-human HGF polyclonal antibody for 24 hours at 4℃. After washing, HRP-conjugated secondary antibody (1:10,000 dilution) was added. Binding was confirmed using an enhanced chemiluminescence system (ECL, Amersham), and subsequent X-ray film development.
The total RNA was isolated with TRI reagent according to the protocol recommended by the manufacturer, and 1 µg of RNA was used for the first strand of cDNA systhesis. The resulting RT products were amplified under the following conditions: 94℃ for 5 minutes, followed by 30 cycles at 94℃ for 30 seconds, at 55℃ for 1 minutes, and at 72℃ for 30 seconds, and followed by final cycles at 72℃ for 10 minutes. The amplification products were separated on 1% agarose gels and visualized by ethidium bromide staining. The following sets of primers were used: HGF forward primer 5'- ATC- CTC ATG GAC CCT GGT G -3'; and HGF reverse primer 5'- GGC CTG GCA AGC TTC ATT A-3'; c-met/HGF-R forward primer 5'- AGA TCA TCC ATT GCA TTC GA -3'; and c-met/HGF-R reverse primer 5'- TGA CGA TCT TGT TGA AGA AG -3'.
Southern blot analysis of the PCR products was conducted by denaturation of the DNA in the gel with denaturation buffer [1.5 M NaCl, 0.5 M NaOH] for 45 minutes. This was followed by neutralization in buffer twice [1 M Tris (pH 7.4), 1.5 M NaCl] for 15 minutes each time. The DNA was transferred onto a nylon membrane by capillary action in 20 × SSC and bound by an ultraviolet cross-linking machine, UV StratalinkerTM (Stratagene, La Jolla, CA). The filter wasprehybridized for 1 hour at 42℃ in Dig Easy Hyb Granule (Roche). Hybridization was performed overnight at 42℃. The probes were prepared using a dig-labeling kit by following the manufacturer's instruction. The filters were washed twice for 15 minutes in 0.5×SSC and 0.1% SDS at 42℃, followed by a 30 minutes wash in washing buffer [0.1 M maleic acid, 0.15 M NaCl (pH 7.5), 0.3% Tween-20] at room temperature. Detection of the probes was accomplished using antidigoxigenin-alkaline phosphatase Fab fragments and the chemiluminescence alkaline phosphatase substrate CSPD-star ready to use. The blots were exposed to Kodak X-OmatTM AR film for various lengths of time and the films were then developed. Each analysis was repeated at least three times.
Cells were seeded in 6-well plates (2×105/well) and cultured for 16 hours with DMEM-F12 medium containing 10 % FBS. The cells were then gently washed at least twice with serum-free medium and cultured for 1, 3 or 5 days under serum-free conditions. The cells were harvested by trypsinization, and the total number of cells was counted using a hemocytometer.
RPE cells (5×103/100 µl) were seeded in a 96-well plate and incubated for 16 hours to perform MTS cell proliferation assay. Culture medium was removed and the cells were cultured in serum-free medium for 24 hours. HGF (0.01, 0.1, 1, 10, 50 ng/ml) was added and the cells were further incubated for 48 hours under serum-free conditions. HGF was not added to the control group. Cell Titer 96 Aqueous1 solution (Promega, Madison, WI., USA) was used for cell proliferation assay. 20 µl of cell titer solution was added to each well and allowed to react for 1-4 hours at 37℃. Absorbance at 490 nm was measured on a micro-enzymelinked immunosorbent assay (ELISA) plate reader.
The growth rate of serum-starved RPE cells was equal or increased compared to that of ARPE-19 cells in normal culture conditions (Fig. 1A and B). The cells were scattered and spindle-like in shape (Fig. 1C and D) after starvation.
Expression of HGF and c-met mRNA in normal or stress-induced ARPE-19 cells was determined using RT-PCR (Fig. 2A). The primers were designed to amplify a region of the c-met and HGF mRNA from 820 to 1230, and from 809 to 1324, respectively, thus yielding PCR products of 410 and 515 bp, respectively. High levels of c-met and HGF mRNA expression in serum-starved ARPE-19 cells were detected. With addition of serum, c-met mRNA was expressed faintly, and HGF was not detected. Expression of β-actin mRNA as an internal control was approximately the same at all conditions.
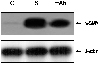
Western blotting of the total cell lysates were carried out (Fig. 2B) to confirm expression of c-Met and HGF in serum starved ARPE-19 cells. A protein of approximately the same size (145 kD) to that of the α-chain of the c-Met was observed. PCR amplification of the cDNA from serumstarved ARPE-19 cells were similar to the patterns of the c-Met expression from western blot. A band of protein corresponding to the size of HGF was not present in the Western blot after incubating with anti-HGF antibody. HGF may have been immediately secreted from the intracellular portion into the extracelluar portion after translation, so HGF expression was evaluated in the concentrated media that were harvested after serum starvation for one to seven days.
Immunoreactive HGF was detected in the conditioned media of cells after serum starvation and it increased in a time-dependent manner (Fig. 2C). The band was approximately 69 kDa in size, representing the active form that is the α-chain of the HGF protein.
MTS proliferation assay with or without treatment of recombinant HGF was performed to evaluate the relationship between the induction of HGF/c-Met expression and abnormal proliferation of ARPE-19 cells after serum starvation.
HGF stimulated ARPE-19 cell proliferation in a dosedependent manner (Fig. 3A). Cell proliferation was increased up to 42% (p<0.01) at 50 ng/ml compared to the control. Neutralizing antibody against c-Met was used to confirm whether the induced HGF/c-Met expression precedes ARPE-19 proliferation. Anti-c-Met antibody (2 ug/ml) inhibited cell proliferation up to 55% (p<0.01, Fig. 3B). It also down-regulated HGF/c-Met mRNA and its protein. These results suggest that HGF/c-Met has an important role on abnormal proliferation of ARPE-19 cells (Fig. 3C and D).
The possibility of RPE cell metaplasia under serum-starved conditions was evaluated. Among various factors related to the transdifferentiation of RPE cells, α-smooth muscle actin (α-SMA), a fibrotic marker protein was used in the study of epithelial-mesenchymal transition (EMT).23
α-SMA was significantly up-regulated at the mRNA level by serum starvation, while it was somewhat down-regulated by blocking with c-Met antibody (Fig. 4). These results suggest that serum starvation enhances the expression of mRNA encoding α-SMA, and are related with the EMT mechanism of the HGF/c-Met signaling pathway.24,25
In this study we demonstrated the importance of HGF /c-Met in ARPE-19 cell proliferation. HGF possesses mitogenic, motogenic and morphogenic properties, and it has been implicated in several retinal diseases, but its roles and properties in the RPE cell are still poorly understood.
We anticipated that stress in the form of serum starvation would induce abnormal proliferation or certain morphological changes on ARPE-19 cells, because they are activated after injury resulting in abnormal proliferation and morphological change to a fibrotic status.1,2,4 Serum starvation usually leads to cell-cycle arrest in the G0-G1 phase and/or apoptosis after prolonged incubation.26-30 The ARPE-19 cells in our study showed abnormal proliferation in a time-dependent manner, but did not proceed to cell death or apoptosis.
HGF is considered to be an important trigger for wound healing or protection against the risk of apoptosis or necrosis in wounded RPE cells or the ischemic retina.20,31 It may have a similar role in serum-starved ARPE-19 cells. RPE cells seem to secrete HGF, among a variety of cytokines and growth factors, in order to maintain retinal homeostasis and visual function or to protect itself or adjacent cells from injury. However, these actions may be of no help, on the contrary, they may induce the development of retinal diseases such as PVR. RPE cells proliferate and migrate from their monolayer area after ocular trauma or rhegmatogenous retinal detachment (RRD), and form sheets of dedifferentiated cells within a provisional extracellular matrix (ECM) that contains fibronectin and thrombospondin.32-34
HGF and c-met mRNA are reported to be expressed from cultured primary RPE cells. This may reflect the fact that the cultured status itself is a form of stress. Abnormal proliferation of ARPE-19 cells and induction of HGF/c-Met expression during serum starvation suggest that activation of RPE cells is related with HGF/c-Met in the non-physiologic condition. Furthermore, an elevated level of HGF allows ARPE-19 cells to resist cell death or stress during cell starvation. We propose that stress may not only cause an abnormal life cycle for ARPE-19 cells, but it may also trigger the cellular reactions leading to HGF/c-Met expression. Neutralization experiment against c-Met on serum-starved ARPE-19 cell was performed to prevent HGF from binding to its receptor. Neutralization inhibited RPE cell proliferation, and also down regulation of c-Met expression, thus confirming that the c-Met/HGF pathway is strongly related to RPE cell proliferation. It also decreased the stress-induced expression of HGF transcripts. This strongly suggests that during starvation, initial activation of c-Met is required for the subsequent full expression of HGF transcripts.
Stress caused by serum starvation may induce abnormal proliferation of RPE cells, and may further be related to RPE metaplasia. The significant expression of αSMA in serum-starved ARPE-19 cells shown in the present study adds weight to the suggestion that Met/HGF may play a key role in the epithelial-to-mesenchymal shift of RPE cells. α-SMA, a fibrosis marker is known to be expressed in late passages of primary cultured RPE cells and transdifferentiation of RPE cells into myofibroblasts can be assessed by the quantitation of α-SMA27 Guidry et al were able to show that RPE can transdifferentiate with upregulation of α-SMA expression in age-related macular degeneration.35 Increased expression of α-SMA in serum-starved ARPE-19 cells and decreased expression in neutralization tests show that RPE metaplasia and HGF/c-Met pathway form a loop, although these results are unique for the transdifferentiation of RPE cells.
Down-stream signaling pathway of HGF/c-Met on stress-induced RPE cells in vitro and subsequent effects on a PVR animal model in vivo are further required in the future.
Figures and Tables
Fig. 1
ARPE-19 cell proliferation patterns and scattering during serum-starvation. Serum-starved ARPE-19 cells were counted using a hemocytometer (A). MTS assay was performed at 490 nm on a micro-enzyme-linked immunosorbent assay (ELISA) plate reader (B). ARPE-19 cells showed scattering pattern and spindle shape after serum starvation for 24 hours (C) and (D).

Fig. 2
Induction of HGF and HGF-R/c-met expression on serum-starved ARPE-19 cells, as determined by RT-PCR analysis (A) and Western blot (B) and (C). A. After serum starvation for 24 hours, RT-PCR using HGF and HGF-R primer was performed. We detected extremely high levels of c-met and HGF mRNA expression in serum-starved ARPE-19, but only a faint level of c-met mRNA expression in normal ARPE-19 cells was seen. B. To confirm the effect of stress, expression of c-Met proteins in serum-starved ARPE-19 cells was examined using Western blot. The results of western blot from stress-induced ARPE-19 are similar with those of PCR amplification of the cDNA (A). C. HGF expression was not detected from the total cell lysates, but it was detected from the concentrated media. The active form of HGF, mature HGF (69 kD), was detected after serum starvation for several days in concentrated media.
*represents serum starvation periods. C; Normal ARPE-19 cell as a control, S; serum deprivation for 24 hours.

Fig. 3
A. Effect of HGF on proliferation of ARPE-19. After serum starvation for 24 hours, HGF at 0.01, 0.1, 1, 10, 50 ng/ml
concentrations were added and incubation was done for 48 hours under serum-free conditions. HGF stimulated ARPE-19 cell proliferation in a dose-dependent manner. (n=9, S.D. 3.7%, p<0.01) B. Inhibition of ARPE-19 cell proliferation with neutralizing the HGF receptor/c-Met. To investigate the relationship between the induction of c-met, HGF expression and serum-starved ARPE-19 proliferation, we performed a colorimetric procedure to the known MTS proliferation assay. Neutralized ARPE-19 cells with anti-c-met antibody (2 µg/ml) inhibited cell proliferation up to 55%. A and B data are presented as means±S.D, n=9. C and D. Down regulation of HGF/c-Met in ARPE-19 cells with neutralizing c-Met antibody. After neutralization, HGF was down-regulated at the transcriptional level (C), but c-Met was at translational level (D). C; normal ARPE-19 as a control, S; after serum starvation for 24 hours, PBS was added for 2 days as a control of mAb, S+mAb; after serum starvation for 24 hours, neutralization was performed using monoclonal anti-mouse c-Met
antibody (mAb) for 2 days.

Fig. 4
Expression pattern of the α-SMA by stress induction or neutralization against c-Met. RT-PCR and Southern blot analysis of α-SMA expression in ARPE-19 cells. C; normal ARPE-19 as a control, S; after serum starvation for 24 hours, normal mouse IgG was added for 2 days as a control of mAb, S+mAb; after serum starvation for 24 hours, neutralization was performed using monoclonal anti-mouse c-Met antibody (mAb) for 2 days.
References
1. Machemer R, Aaberg TM, Freeman HM, et al. An updated classification of retinal detachment with proliferative vitreoretinopathy. Am J Ophthalmol. 1991. 112:159–165.
2. Grierson I, Heathcote L, Hiscott P, et al. Hepatocyte growth factor/scatter factor in the eye. Prog Retin Eye Res. 2000. 19:779–802.
3. Kon CH, Occleston NL, Aylward GW, Khaw PT. Expression of vitreous cytokines in proliferative vitreoretinopathy: a prospective study. Invest Ophthalmol Vis Sci. 1999. 40:705–712.
4. Boros P, Miller CM. Hepatocyte growth factor: a multifunctional cytokine. Lancet. 1995. 345:293–295.
5. Chirgadze DY, Hepple J, Byrd RA, et al. Insights into the structure of hepatocyte growth factor/scatter factor (HGF/SF) and implications for receptor activation. FEBS Lett. 1998. 430:126–129.
6. Mars WM, Zarnegar R, Michalopoulos GK. Activation of hepatocyte growth factor by the plasminogen activators uPA and tPA. Am J Pathol. 1993. 143:949–958.
7. Nakamura T, Nishizawa T, Hagiya M, et al. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989. 342:440–443.
8. Pagan R, Martin I, Llobera M, Vilaro S. Growth and differentiation factors inhibit the migratory phenotype of cultured neonatal rat hepatocytes induced by HGF/SF. Exp Cell Res. 1997. 235:170–179.
9. Bussolino F, Di Renzo MF, Ziche M, et al. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol. 1992. 119:629–641.
10. Komada M, Kitamura N. The cell dissociation and motility triggered by scatter factor/hepatocyte growth factor are mediated through the cytoplasmic domain of the c-Met receptor. Oncogene. 1993. 8:2381–2390.
11. Bhargava M, Joseph A, Knesel J, et al. Scatter factor and hepatocyte growth factor: activities, properties, and mechanism. Cell Growth Differ. 1992. 3:11–20.
12. Longati P, Comoglio PM, Bardelli A. Receptor tyrosine kinases as therapeutic targets: the model of the MET oncogene. Curr Drug Targets. 2001. 2:41–55.
13. Niemann C, Brinkmann V, Spitzer E, et al. Reconstitution of mammary gland development in vitro: requirement of c-met and c-erbB2 signaling for branching and alveolar morphogenesis. J Cell Biol. 1998. 143:533–545.
14. Sonnenberg E, Meyer D, Weidner KM, Birchmeier C. Scatter factor/hepatocyte growth factor and its receptor, the c-met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. J Cell Biol. 1993. 123:223–235.
15. Tsarfaty I, Rong S, Resau JH, et al. The Met proto-oncogene mesenchymal to epithelial cell conversion. Science. 1994. 263:98–101.
16. Weidner KM, Sachs M, Birchmeier W. The Met receptor tyrosine kinase transduces motility, proliferation, and morphogenic signals of scatter factor/hepatocyte growth factor in epithelial cells. J Cell Biol. 1993. 121:145–154.
17. Briggs MC, Grierson I, Hiscott P, Hunt JA. Active scatter factor (HGF/SF) in proliferative vitreoretinal disease. Invest Ophthalmol Vis Sci. 2000. 41:3085–3094.
18. Jin M, Chen Y, He S, et al. Hepatocyte growth factor and its role in the pathogenesis of retinal detachment. Invest Ophthalmol Vis Sci. 2004. 45:323–329.
19. Liou GI, Pakalnis VA, Matragoon S, et al. HGF regulation of RPE proliferation in an IL-1beta/retinal hole-induced rabbit model of PVR. Mol Vis. 2002. 8:494–501.
20. Shibuki H, Katai N, Kuroiwa S, et al. Expression and neuroprotective effect of hepatocyte growth factor in retinal ischemia-reperfusion injury. Invest Ophthalmol Vis Sci. 2002. 43:528–536.
21. Umeda N, Ozaki H, Hayashi H, et al. Non-paralleled increase of hepatocyte growth factor and vascular endothelial growth factor in the eyes with angiogenic and nonangiogenic fibroproliferation. Ophthalmic Res. 2002. 34:43–47.
22. Lashkari K, Rahimi N, Kazlauskas A. Hepatocyte growth factor receptor in human RPE cells: implications in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 1999. 40:149–156.
23. Rosen EM, Knesel J, Goldberg ID, et al. Scatter factor modulates the metastatic phenotype of the EMT6 mouse mammary tumor. Int J Cancer. 1994. 57:706–714.
24. Grimm S, Bauer MK, Baeuerle PA, Schulze-Osthoff K. Bcl-2 down-regulates the activity of transcription factor NF-kappaB induced upon apoptosis. J Cell Biol. 1996. 134:13–23.
25. Rosen EM, Nigam SK, Goldberg ID. Scatter factor and the c-met receptor: a paradigm for mesenchymal/epithelial interaction. J Cell Biol. 1994. 127:1783–1787.
26. Bissonnette N, Hunting DJ. p21-induced cycle arrest in G1 protects cells from apoptosis induced by UV-irradiation or RNA polymerase II blockage. Oncogene. 1998. 16:3461–3469.
27. Hasan NM, Adams GE, Joiner MC. Effect of serum starvation on expression and phosphorylation of PKC-alpha and p53 in V79 cells: implications for cell death. Int J Cancer. 1999. 80:400–405.
28. Miura Y, Yanagihara N, Imamura H, et al. Hepatocyte growth factor stimulates proliferation and migration during wound healing of retinal pigment epithelial cells in vitro. Jpn J Ophthalmol. 2003. 47:268–275.
29. Park CH, Kim HR, Kim J, et al. Latent membrane protein 1 of Epstein-Barr virus plays an important role in the serum starvation resistance of Epstein-Barr virus-immortalized B lymphocytes. J Cell Biochem. 2004. 91:777–785.
30. Yoshida M, Beppu T. Reversible arrest of proliferation of rat 3Y1 fibroblasts in both the G1 and G2 phases by trichostatin A. Exp Cell Res. 1988. 177:122–131.
31. Machemer R, Van Horn D, Aaberg TM. Pigment epithelial proliferation in human retinal detachment with massive periretinal proliferation. Am J Ophthalmol. 1978. 85:181–191.
32. Hiscott P, Sheridan C, Magee RM, Grierson I. Matrix and the retinal pigment epithelium in proliferative retinal disease. Prog Retin Eye Res. 1999. 18:167–190.
33. Lee SC, Kwon OW, Seong GJ, et al. Epitheliomesenchymal transdifferentiation of cultured RPE cells. Ophthalmic Res. 2001. 33:80–86.
34. Scheiffarth OF, Kampik A, Gunther H, Von der mark K. Proteins of the extracellular matrix in vitreoretinal membranes. Graefes Arch Clin Exp Ophthalmol. 1988. 226:357–361.
35. Guidry C, Medeiros NE, Curcio CA. Phenotypic variation of retinal pigment epithelium in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2002. 43:267–273.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download