Abstract
Objective
The beneficial effect of decompressive craniectomy in the treatment of severe traumatic brain injury (TBI) is controversial, but there is no debate that decompression should be performed before irreversible neurological deficit occurs. The aim of our study was to assess the value of ultra-early decompressive craniectomy in patients with severe TBI.
Methods
Total of 127 patients who underwent decompressive craniectomy from January 2007 to December 2013 was included in this study. Among them, 60 patients had underwent ultra-early (within 4 hours from injury) emergent operation for relief of increased intracranial pressure. Initial Glasgow coma scale, brain computed tomography (CT) scan features by Marshall CT classification, and time interval between injury and craniectomy were evaluated retrospectively. Clinical outcome was evaluated, using the modified Rankin score.
Results
The outcomes of ultra-early decompressive craniectomy group were not better than those in the comparison group (p=0.809). The overall mortality rate was 68.5% (87 patients). Six of all patients (4.7%) showed good outcomes, and 34 patients (26.8%) remained in a severely disabled or vegetative state. Forty of sixty patients (66.7%) had died, and two patients (3.3%) showed good outcomes at last follow-up.
Over 60% of severe traumatic brain injury (TBI) patients are known to die or survive but with severe disability. High intracranial pressure (ICP) or malignant brain swelling, proved by clinical and/or radiological point of view is highly associated with poor outcomes and account for majority of deaths in TBIs (79.2%).5,11,17) To control the high ICP, both medical and surgical treatment-decompressive craniectomy-can be performed. As the latter is known to provide significant improvement over cerebral compliance, cerebral blood flow (CBF) and oxygenation with consequent improvement of the patient's clinical outcome, when medical treatment is considered futile, operation if performed to manage the high ICP.
To the best of our knowledge, there are few previous reports comparing clinical outcomes of early to delayed decompressive craniectomy after TBI and there is no set consensus on optimal time for surgery. In suggestion that differences in surgical time could be the major factor affecting mortality, we retrospectively analyzed the overall results of 127 patients with severe TBI according to surgical time at a single institution.
A retrospective review of data was performed using a medical database of patients who had undergone decompressive craniectomy at the authors' institution between January 2007 and December 2013. A total of 1,896 head-injured patients over 18 years old was admitted during this period. Brain computed tomography (CT) scans were taken on admission, and of them 127 cases (6.7%) were found to have traumatic intracranial hemorrhage or severe brain swelling that required emergent decompressive craniectomy.
Subjects who fulfilled the following inclusion criteria were evaluated: over age of 18, with blunt head trauma, a "severe TBI" patients with Glasgow coma scale (GCS)23) of 3-8 at admission. Patients with hypotension (systolic blood pressure <90 mm Hg), hypoxia (PaO2 <70 mm Hg), dead on arrival, whole brain ischemia (black brain), penetrating injury, and concomitant life-threatening medical condition unlikely to survive from craniectomy were excluded. Collected clinical profile for this study included initial GCS score, patient demographics, injury mechanism, vital sign at emergency room, CT scans, intracranial pathological finding, surgical procedure, intraoperative findings, treatment outcome, and the time interval between injury and craniectomy. For evaluation of the brain CT, septum pellucidum was assigned as the midline structure, and the brain shifting was recorded on a 3-point scale as <10 mm, 10-20 mm, or >20 mm by measuring the longest distance from the midline to the shifted brain parenchyme. Marshall CT classification was also used for additional classification. Neurological outcome was evaluated following the modified Rankin scale (mRS).19) The six mRS categories (good, mild disabled, moderately disabled, severely disabled, vegetative, dead) were reduced into three groups-favorable, unfavorable and dead-for data analysis and a favorable outcome was defined as a mRS of 0-2.
Time to Surgery was defined as the duration from the onset of the trauma to the moment of the skin incision in the operating room, with Ultra Early Surgery defined as Time to Surgery within 4 hours of the trauma event onset. Patients were selected at random. All patients were operated on by using the same surgical technique: those with diffuse cerebral edema without midline shifting received bilateral craniectomy, and those who presented with midline shifting and mass effect by intracranial hemorrhage received ipsilateral unilateral craniectomy with combined duraplasty using artificial dura. During the procedure, temporal bone was removed as close to the base as possible to decompress the brain stem. The cases with elevated ICP without massive intracranial hemorrhage were treated additionally by mannitol administration, cerebrospinal fluid drainage, barbiturate coma therapy, or hypothermia when indicated. SPSS version 18 was used for statistical analyses (SPSS Inc., Chicago, IL, USA). Statistical analyses were performed by use of t-tests and one-way analyses of variance for continuous variables, and χ2 or Fisher's exact test for categorical variables. Differences were considered statistically significant if p-values were less than 0.05.
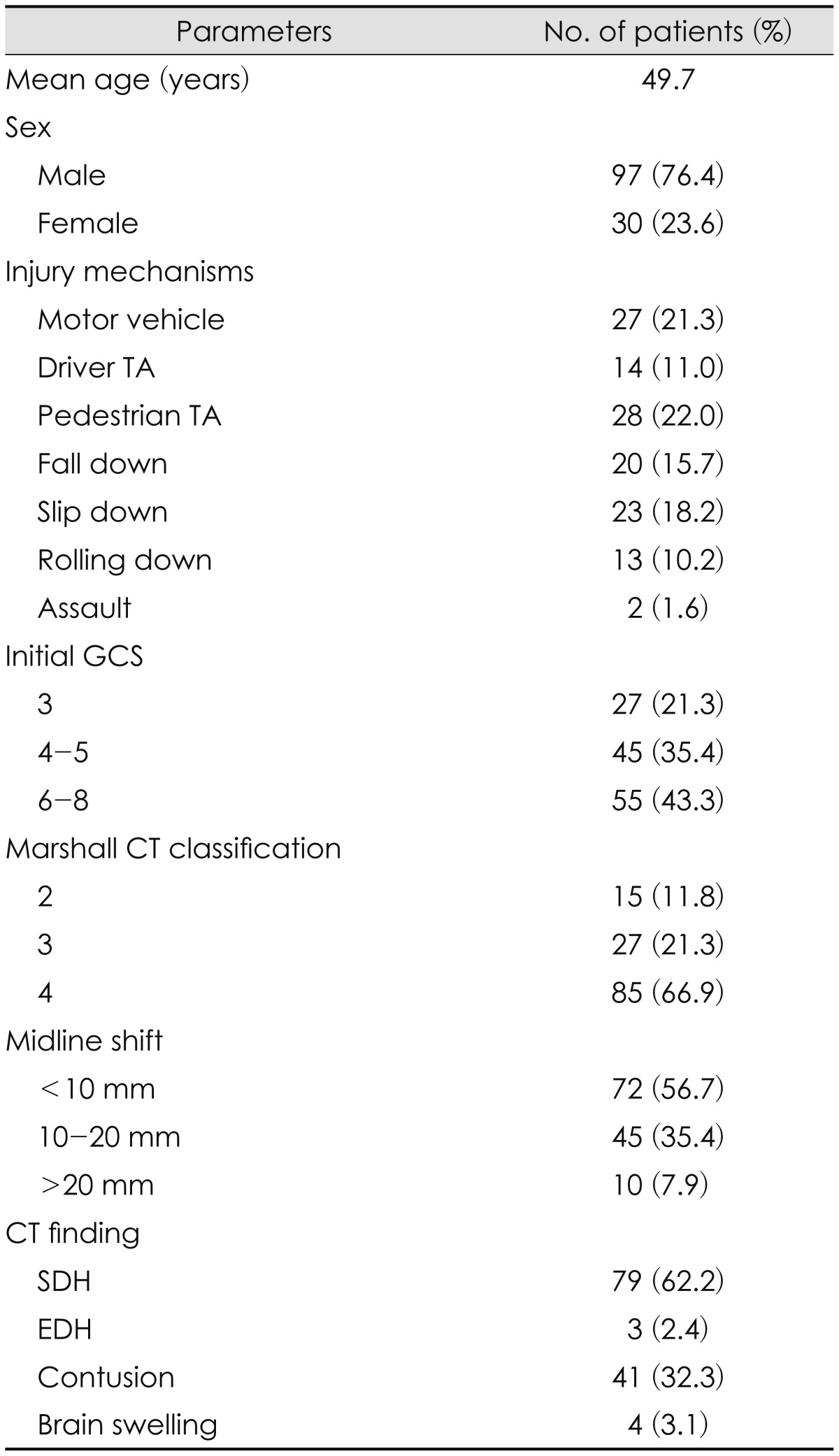
Patient data for severe TBI are described in Table 1. Most of the patients (97 out of 127) were males, with mean age of 49.7 years (range, 18-90 years), and injury mechanisms showed even distribution overall.
Neurological evaluation at the time of admission was performed using the GCS. Twenty seven patients had a GCS of 3 and after having received decompressive craniectomy, all had died. Forty-five patients had a GCS of 4 and 5, and the mortality rate was 82.2% for this group. Fifty-five patients had a GCS of greater or equal to 6 and 37 patients (41%) had died.
Eighty-five patients (66.9%) were consistent with Marshall CT classification 4, with majority of them diagnosed of pure subdural hemorrhage (SDH) with 79 patients (62.2%), and next common group was patients diagnosed of SDH or epidural hemorrhage with contusion and brain laceration, with number of forty-one patients (31.3%).
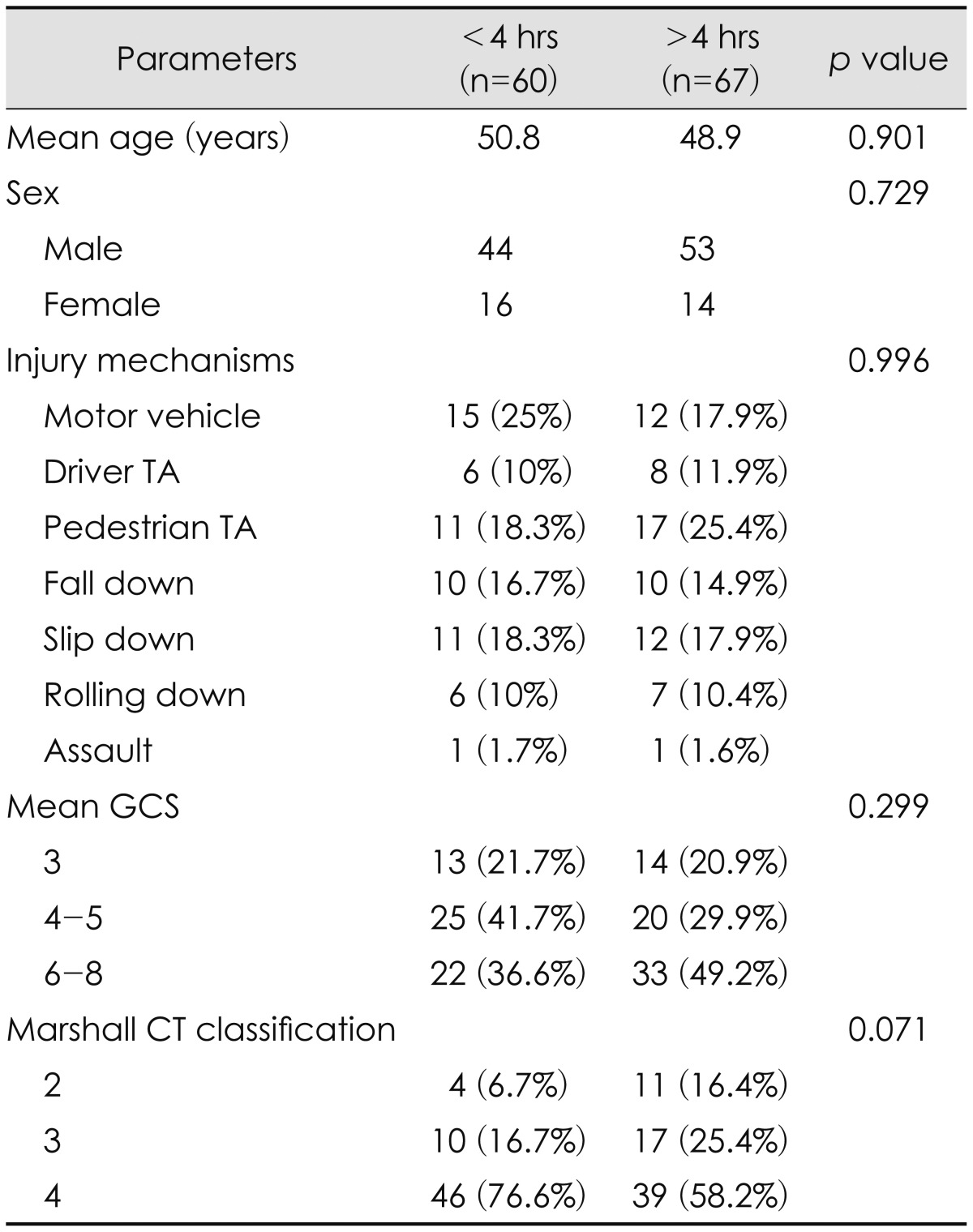
Sixty patients (47.2%) underwent ultra early surgical decompression after trauma (1 hr 53 min-3 hr 59 min, mean 2 hr 59 minutes). In 67 patients (52.8%), surgical decompression was performed after 4 hours of injury (4 hr-27 hr 13 min, mean 7 hr 41 minutes).
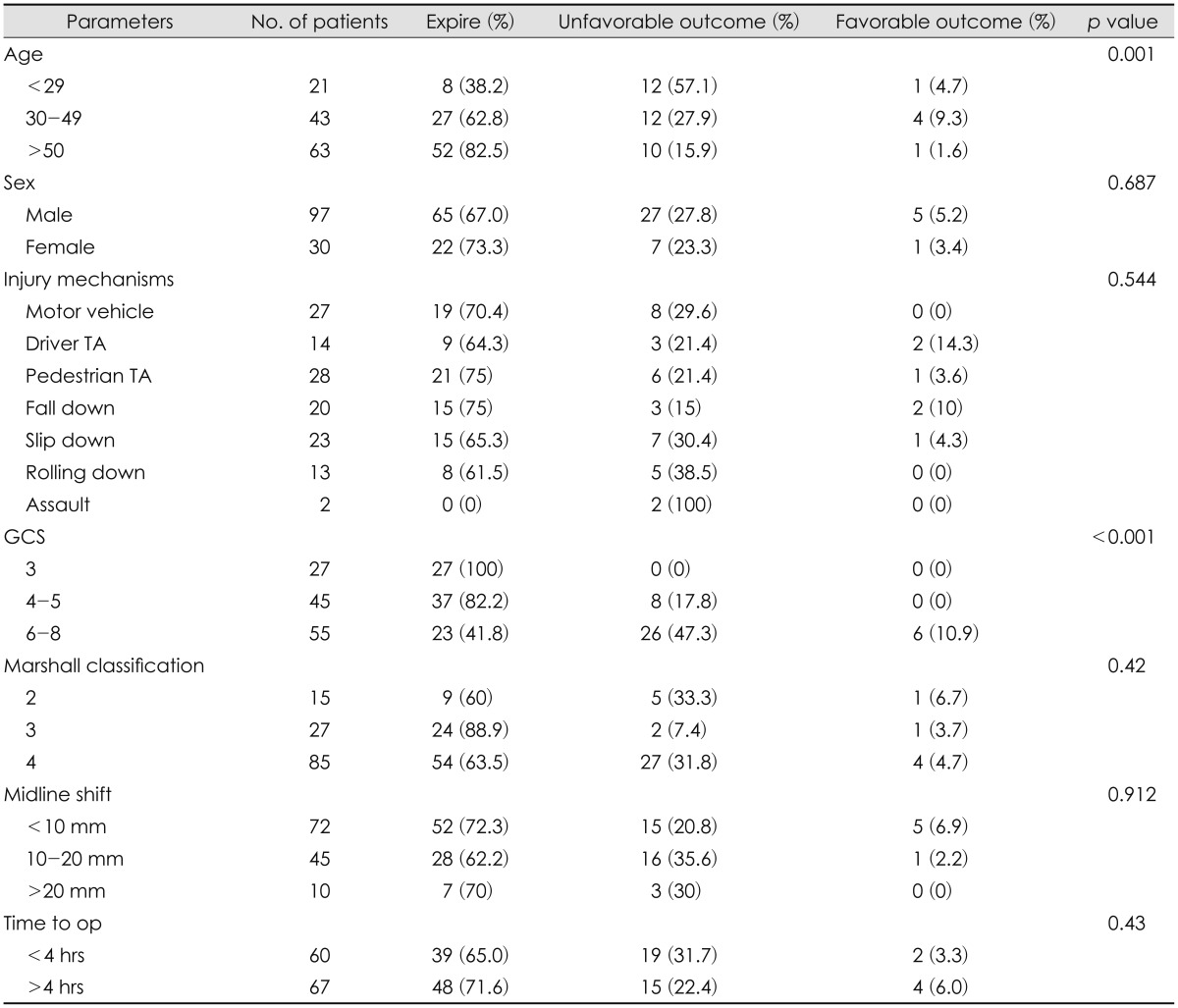
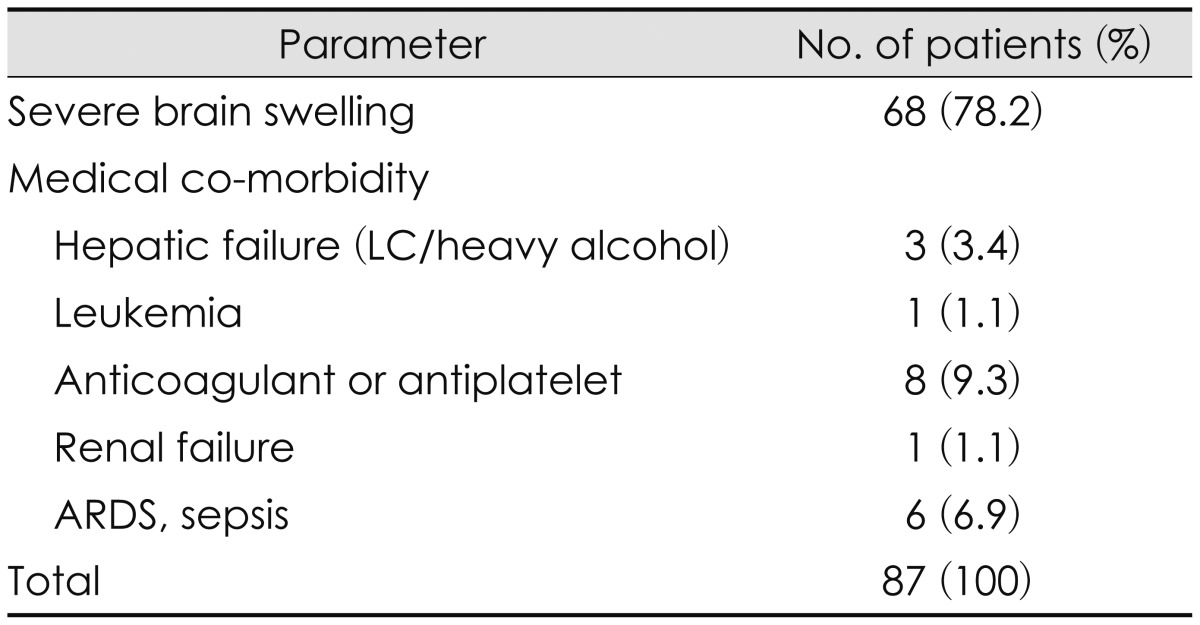
No statistically significant difference of age, sex, or initial GCS between ultra-early and early or delayed treatment groups could be identified. Although ultra-early group tend to show more portion of Marshall CT classification 4 than other, this finding was also not statistically significant (Table 2). Analysis of the overall clinical outcomes revealed that the younger age and lower initial GCS contributed to good outcome, but gender, Marshall CT classification, degree of midline shifting and injury mechanisms did not much affect the outcome (Table 3). Ultra-early decompressed group showed a tendency to have less mortality rate than the other group, but this too had no statistical significance (65.0% vs. 71.6%, p value 0.430). Of the total 127 TBI patients, only 6 patients showed favorable outcomes after decompressive craniectomy, and they had initially presented with GCS over 6. Eighty-seven patients had expired after the surgery, and of them sixty-eight (78.2%) died due to severe brain swelling (Table 4).
TBI patients show poor outcome due to massive direct parenchymal injury and initial insults such as shearing injury of the axons, but this is not the only pathophysiology. The insulted area further get injured by hemorrhage and accompanying cerebral edema, and the consequent brain swelling may lead to uncontrollable increased ICP status, or mass effect of extra- or intra-cerebral hematoma may increase to exacerbate ischemic injury that would end in poor outcome.4,12,14) Especially in cases of multiple lesion trauma patient with systemic complication, consequent systemic hypoxemia, hypotension, hypercarbia and acidosis may be induced to aggravate brain damage even further.4,14) No effective treatment for axonal shearing or stretching injury has been publicized, but speculating from the observation that pre-operative ICP of 24 mm Hg has effectively been turned down to ICP of 14.6 mm Hg after surgical decompression,1,18) if the intracranial lesion can be removed by decompressive craniectomy before causing irreversible damage to the brain in addition to proper medical management, secondary cerebral ischemic injury by mass effect and/or high ICP is strongly thought considered controllable or preventable.4,14,20) With the initial presenting ICP of between 20 and 40 mm Hg, the perfusion pressure is considered enough for adequate amount of CBF that standard therapy could yield relatively good outcomes, whereas in ICP over 40 mm Hg the treatment is less effective and require vigorous therapy, and in ICP >60 mm Hg most of the patients had expired. Based on these findings, Langfitt and Gennarelli14) had stated that ICP is a crucial factor in predicting clinical outcome.
The reason for performing decompressive craniectomy is to recover cerebral perfusion by surgically expanding intracranial space, consequently maintaining the cerebral perfusion pressure and reducing ICP to minimize secondary damage. There is no consensus as to the optimal time to perform the decompressive craniectomy, but there is no question in that the operation should be performed before irreversible neurological deficit occurs. Some advocates that the onset of brain swelling after TBI is within the first two to three hours,16) and although opinions on the time duration for early surgical decompression may vary, there are many reports which states that by having performed early decompressive craniectomy, expected or initially secondary ischemia or axonal injury by refractory intracranial hypertension was preventable.1,4,21,26)
Albanèse et al.3) recommended early operation for those with GCS lower than 6, with intracranial hematoma, and brain swelling and cerebral herniation, whereas Seelig et al.21) had stated that ultra-early surgery of within 4 hours had showed mortality of only 30%, whereas those over the scope of 4 hours had mortality of over 90%. Similarly, decompressive operation within 8 hours of TBI onset was considered the golden time to gain effect, that time to operation room was emphasized as an important factor for prediction of clinical outcome.1,4) Rubiano et al.20) defined 12 hour point as the timeline for 'early surgery', and 16 severe TBI patients had undergone early surgery, whereas 20 patients first performed conventional approach with ventriculostomy and performed craniectomy after the 12 hour timeline. Those who had received early decompressive craniectomy were found to have less mortality (4 out of 16 vs. 13 out of 20). Akyuz et al.2) had noted that the 40 patients who had received early surgery as first tiers had much more portion of better outcome than the other 36 patients operated as second tier (44.4% vs. 12.5%, p value 0.0018). Münch et al.17) had reported the predictors of good outcome as high GCS, rapid surgical decompression, and young age, and of them early surgical decompression definitely improved the midline shifting (-4.8±6.0 mm vs. -0.4±6.3 mm) and mesencephalic cistern decompressed status, and the clinical outcomes evaluated 6 months thereafter showed significant better results in the early decompressed group than the other group. However, the proper timing and outcome after decompressive craniectomy in patients of severe TBI remain controversial. Our study showed that timing of operative intervention for decompression with regard to outcome was not statistically significant. Kotwica and Brzeziński13) and Stone et al.22) and several other authors have concluded that benefits in the early decompressed group do not differ much from that of the late group.26,27) In case of acute subdural hematoma, the shear force at the time of trauma cause damage to the cerebral vasculature and lead to immediate ischemic brain damage, which subsequently lead to acute cerebral swelling, which is probably why concomitant cerebral and vascular lesion damaged at the time of trauma cannot be recovered even if proper management and intervention is introduced in early phase.15) At the initial stage of ischemia, when blood-brain barrier is relatively intact, the hydrostatic pressure caus-ed by interstitial pressure gradient between arterial blood pressure and brain tissue can let the water pass through the endothelium, but early decompressive craniectomy could decrease such interstitial fluid pressure and lead to consequent hydrostatic pressure, further exacerbating brain swelling.9) Some author says that early decompressive surgery may induce infarcts with hemorrhagic transformation that could result in unfavorable outcome.6)
Factors that are most well-known for TBI patients' outcome prediction are GCS and age. Ucar et al.24) have studied outcomes of 100 severe TBI patients depending on initial GCS, and the group with GCS 4-5, 96.6% had showed unfavorable outcome, and the group with GCS 6-8, 65% had showed unfavorable outcome. As 87.5% of the group with favorable outcome was GCS 6 to 8, he concluded that clinical outcome is well determined by initial GCS. Our hospital also showed mortality rate of 100%, 82.2%, 41.8% for each respective GCS group GCS 3, 4-5, 6-8, and whereas those under GCS 5 never showed any favorable outcome, those over GCS 6 (two each from GCS 6, 7, 8) showed a portion of 10.9% (p<0.001). Therefore, we concluded that we can expect to have a relatively favorable outcome in those over GCS 6 with lower mortality, and that initial GCS is a good predictor of clinical outcome. Many authors have reported of the relationship between old age and high mortality.13,27) Howard et al.10) had reported a mortality rate of 74% in those over 65 years old, in contrast to 18% mortality rate in those aged between 18 to 40, and Wilberger et al.27) had also showed higher mortality rate in the over 65-year-old group with statistical significance (82% vs. 54%). Our hospital yielded results similar to the mentioned studies. Mortality rate was 38.2% in the patients under the age of 29 years and 82.5% mortality rate was observed in patients over 50. Howard et al.10) has explained that the elderly patients tend to be exposed to more destructive injury, the elderly often have as much as 4 times the volume of control group acute SDH, resulting in about twice the midline shifting. Also, for aging brains have less neurotrophic factors secreted after brain injuries compared to the younger brains, with subsequent regenerative capacity decrement and speculated this is why the older age patients tend to have higher mortality rate. There have been people on the other side as well.8) Aarabi et al.1) underwent retrospective evaluation of 50 patients on age, GCS, papillary response, midline shift, timing of decompressive craniectomy-and had concluded that none of these factors have any statistical significance.
Wilberger et al.27) have stated that the motorcycle accidents, which showed the highest mortality of 71% among TBI patients, may have to do with concomitant cerebral damage related to high velocity injury, especially impact damages such as cortical contusions, lacerations, bone fragmentation, diffuse axonal injury, and brainstem injuries. According to our data, although injury due to fall down and driver traffic accident had relatively high favorable outcome, the sample size was too small to have any statistical power and as the result, injury mechanism did not have statistically significant meaning in relation to mortality rate (p=0.544).
Some have studied the relationship between the CT finding and its outcome. Kotwica and Brzeziński 13) have reported that although 85% of patients with large unilateral brain contusion have died, only 17% of those with small lesion have died, and that the severity of midline shifting is associated with worse outcome. But data in our hospital, based on the category of midline shifting under 10 mm, 10-20 mm, over 20 mm, did not show statistical significance (p=0.912). Also, there have been reports that compression or obliteration of mesencephalic cistern, or small sized or asymmetric ventricle can be associated with brain swelling and herniation that these could be predictors for outcomes after TBI.4,7,25) In our study, favorable outcome based on Marshall CT classification showed statistical significance (p=0.021), consistent with above mentioned report. Therefore, rather than just making predictions base on solitary midline shifting, the compression status of mesencephalic cistern by the high ICP-which is, the herniation of brainstem-seems to be the more important predicting factor.
One of the advantages of this study is the relatively large cohort. However, its retrospective characteristic led to less complete and less accurate data on the rebound. Cohort was still not large enough to represent all of the patients' results, and post-operative ICP control status was uncheckable for not every patient had undergone perioperative ICP measurement. Furthermore, we had not so small portion of patients who presented with a GCS of 3 and fatal pons sign of bilateral dilated pupils at the time of admission, which may have contributed to relatively higher mortality.
Patients who undergo decompressive craniectomy within the first 4 hours following trauma may not have better outcomes compared to those who undergo decompressive craniectomy after 4 hours. Patient factors such as initial GCS and age may be the most important factors affecting the outcome, rather than factors such as time taken for decompression. Therefore, strict application of surgery within the first 4 hours of TBI onset does not seem mandatory.
References
1. Aarabi B, Hesdorffer DC, Ahn ES, Aresco C, Scalea TM, Eisenberg HM. Outcome following decompressive craniectomy for malignant swelling due to severe head injury. J Neurosurg. 2006; 104:469–479. PMID: 16619648.

2. Akyuz M, Ucar T, Acikbas C, Kazan S, Yilmaz M, Tuncer R. Effect of early bilateral decompressive craniectomy on outcome for severe traumatic brain injury. Turk Neurosurg. 2010; 20:382–389. PMID: 20669113.

3. Albanèse J, Leone M, Alliez JR, Kaya JM, Antonini F, Alliez B, et al. Decompressive craniectomy for severe traumatic brain injury: Evaluation of the effects at one year. Crit Care Med. 2003; 31:2535–2538. PMID: 14530763.

4. Cavuşoğlu H, Kaya RA, Türkmenoğlu ON, Aydin Y. Value of early unilateral decompressive craniectomy in patients with severe traumatic brain injury. Ulus Travma Acil Cerrahi Derg. 2010; 16:119–124. PMID: 20517764.
5. Cooper DJ, Rosenfeld JV, Murray L, Wolfe R, Ponsford J, Davies A, et al. Early decompressive craniectomy for patients with severe traumatic brain injury and refractory intracranial hypertension--a pilot randomized trial. J Crit Care. 2008; 23:387–393. PMID: 18725045.

6. Cooper PR, Hagler H, Clark WK, Barnett P. Enhancement of experimental cerebral edema after decompressive craniectomy: implications for the management of severe head injuries. Neurosurgery. 1979; 4:296–300. PMID: 450227.
7. Eisenberg HM, Gary HE Jr, Aldrich EF, Saydjari C, Turner B, Foulkes MA, et al. Initial CT findings in 753 patients with severe head injury. A report from the NIH Traumatic Coma Data Bank. J Neurosurg. 1990; 73:688–698. PMID: 2213158.
8. Haselsberger K, Pucher R, Auer LM. Prognosis after acute subdural or epidural haemorrhage. Acta Neurochir (Wien). 1988; 90:111–116. PMID: 3354356.

9. Hatashita S, Hoff JT. Biomechanics of brain edema in acute cerebral ischemia in cats. Stroke. 1988; 19:91–97. PMID: 3336907.

10. Howard MA 3rd, Gross AS, Dacey RG Jr, Winn HR. Acute subdural hematomas: an age-dependent clinical entity. J Neurosurg. 1989; 71:858–863. PMID: 2585078.

11. Huang YH, Lee TC, Lee TH, Liao CC, Sheehan J, Kwan AL. Thirty-day mortality in traumatically brain-injured patients undergoing decompressive craniectomy. J Neurosurg. 2013; 118:1329–1335. PMID: 23472847.

12. Jagannathan J, Okonkwo DO, Dumont AS, Ahmed H, Bahari A, Prevedello DM, et al. Outcome following decompressive craniectomy in children with severe traumatic brain injury: a 10-year single-center experience with long-term follow up. J Neurosurg. 2007; 106(4 Suppl):268–275. PMID: 17465359.

13. Kotwica Z, Brzeziński J. Acute subdural haematoma in adults: an analysis of outcome in comatose patients. Acta Neurochir (Wien). 1993; 121:95–99. PMID: 8512021.

14. Langfitt TW, Gennarelli TA. Can the out come from head injury be improved? J Neurosurg. 1982; 56:19–25. PMID: 7054418.
15. Massaro F, Lanotte M, Faccani G, Triolo C. One hundred and twenty-seven cases of acute subdural haematoma operated on. Correlation between CT scan findings and outcome. Acta Neurochir (Wien). 1996; 138:185–191. PMID: 8686543.
16. Mathai KI, Sudumbrekar SM, Shashivadhanan , Sengupta SK, Rappai TJ. Decompressive craniectomy in traumatic brain injury rationale and practice. Indian J Neurotrauma. 2010; 7:9–12.

17. Münch E, Horn P, Schürer L, Piepgras A, Paul T, Schmiedek P. Management of severe traumatic brain injury by decompressive craniectomy. Neurosurgery. 2000; 47:315–322. discussion 322-323. PMID: 10942004.

18. Polin RS, Shaffrey ME, Bogaev CA, Tisdale N, Germanson T, Bocchicchio B, et al. Decompressive bifrontal craniectomy in the treatment of severe refractory posttraumatic cerebral edema. Neurosurgery. 1997; 41:84–92. discussion 92-94. PMID: 9218299.

19. Rankin J. Cerebral vascular accidents in patients over the age of 60. II. Prognosis. Scott Med J. 1957; 2:200–215. PMID: 13432835.

20. Rubiano AM, Villarreal W, Hakim EJ, Aristizabal J, Hakim F, Dìez JC, et al. Early decompressive craniectomy for neurotrauma: an institutional experience. Ulus Travma Acil Cerrahi Derg. 2009; 15:28–38. PMID: 19130336.
21. Seelig JM, Becker DP, Miller JD, Greenberg RP, Ward JD, Choi SC. Traumatic acute subdural hematoma: major mortality reduction in comatose patients treated within four hours. N Engl J Med. 1981; 304:1511–1518. PMID: 7231489.
22. Stone JL, Rifai MH, Sugar O, Lang RG, Oldershaw JB, Moody RA. Subdural hematomas. I. Acute subdural hematoma: progress in definition, clinical pathology, and therapy. Surg Neurol. 1983; 19:216–231. PMID: 6836474.
23. Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974; 2:81–84. PMID: 4136544.
24. Ucar T, Akyuz M, Kazan S, Tuncer R. Role of decompressive surgery in the management of severe head injuries: prognostic factors and patient selection. J Neurotrauma. 2005; 22:1311–1318. PMID: 16305319.

25. van Dongen KJ, Braakman R, Gelpke GJ. The prognostic value of computerized tomography in comatose head-injured patients. J Neurosurg. 1983; 59:951–957. PMID: 6631517.

26. Wen L, Wang H, Wang F, Gong JB, Li G, Huang X, et al. A prospective study of early versus late craniectomy after traumatic brain injury. Brain Inj. 2011; 25:1318–1324. PMID: 21902550.

27. Wilberger JE Jr, Harris M, Diamond DL. Acute subdural hematoma: morbidity, mortality, and operative timing. J Neurosurg. 1991; 74:212–218. PMID: 1988590.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download