Abstract
Diffuse or focal white matter hyperintensity lesions on MRI have been reported in only a few patients with Hashimoto's encephalopathy (HE), and anti-TPO antibody level is high in most cases. We report a 59-year-old woman who presented with acute onset of disorientation with confusion. Anti-thyroglobulin antibody was detected in high titer, although anti-TPO antibody titer was not high. Thyroid sonography and biopsy revealed Hashimoto's thyroiditis. Initial fluid-attenuated inversion recovery (FLAIR) image and diffusion-weighted imaging (DWI) revealed ill-defined, diffuse, high-signal intensity lesions on the deep white matters and globus pallidus. Brain SPECT showed significant hypoperfusion in both basal ganglia (especially globus pallidus), frontal and temporal lobes. With the impression of HE, the patient was treated on a high-dose steroid. Over the next 15 weeks, her cognition improved to a nearly normal state and the MRI findings on DWI and FLAIR showed resolution paralleling her clinical improvement. Our case illustrates the peculiar changes in the MR findings, especially in DWI, with hypoperfusion on brain SPECT in patients with HE and allows for a greater understanding of the pathophysiology of HE.
Hashimoto's encephalopathy (HE) is a disease accompanied by various neuropsychological manifestations, including cognition or consciousness deterioration, personality changes, and seizures or hallucinations [1]. Recently, HE has attracted increased attention because it is regarded as a cause of treatable dementia and has also been recognized as one of the important differential diagnoses of Creutzfeldt-Jakob disease. HE often shows increased anti-thyroglobulin (anti-Tg) antibody and anti-thyroperoxidase (anti-TPO) antibody levels and may respond positively to steroid treatment [1]. Although autoimmune reactivities are thought to be an underlying pathomechanism in HE, the etiology of the disease is still incompletely understood. When HE occurs, thyroid hormones are at a normal level or may indicate only a mild decrease in thyroid function. Therefore, an anti-thyroid antibody test should be carried out in addition to a thyroid function test [2].
Brain magnetic resonance imaging (MRI) in HE patients reveals heterogeneous findings, from entirely normal findings to various degrees of nonspecific abnormalities such as focal or diffuse hyperintensities on either side of the brain parenchyma. Few cases have reported serial, diffusion-weighted imaging (DWI) follow-up evaluations in patients with HE [3,4]. We report a distinct case of HE showing global hypoperfusion on brain SPECT and marked improvement on DWI and FLAIR images after high-dose steroid therapy, and we will discuss pathophysiology of Hashomoto's encephalopathy by interpretation of imaging findings of brain SPECT and serial changes of brain MRI in this case.
A 59-year-old female patient presented with feelings of disorientation and confusion over a period of three weeks. She indicated that she was experiencing disorientation of time, vacant feelings, was not speaking much, had reduced memory function, and had a slower gait. There were no other significant diseases indicated in her medical or family history. The patient's physical examination showed a thyroid that was within normal limits of size and hardness; she did not exhibit any signs of illness.
At initial presentation, the patient showed clear consciousness in neurological examination. While her orientation toward place and people was normal, her time orientation was significantly decreased. The Korean version of the mini-mental state examination (K-MMSE) showed a score of 15/30, reflecting a significant impairment. Detailed neuropsychological tests showed multiple cognitive deficits, including memory, orientation to time and place, and frontal executive function. She walked very slowly and showed slight bradykinesia. However, she did not exhibit tremors, spasticity, or other symptoms associated with her extrapyramidal system. In addition, neurological abnormalities such as muscle weakness, abnormal sensation, or ataxia were not observed. Her blood tests demonstrated normal values, as did her thyroid function test. Analysis of her cerebrospinal fluid (CSF) revealed normal levels of sugars and proteins and the absence of inflammatory cells. However, her anti-Tg antibody level showed a significant increase to 334.95 IU/mL (normal≤70 IU/mL) and her anti-TPO antibody level was 34.86 IU/mL (normal≤60 IU/mL), and other antibodies were negative about neutrophil cytoplasmic (ANCA), nuclear (ANA), SSA, SSB, dsDNA, Jo-1 and Scl-70. Ultrasound of her thyroid showed a diffuse, irregular, hypoechoic change. A cone-biopsy of her thyroid gland showed a lymphoid follicle and Hurthle cell changes around the follicular epithelium (Fig. 1), confirming HE.
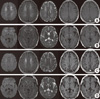
Brain MRI, using DWI, T2-weighted images, and FLAIR, showed a wide array of high-signal intensities, mainly on the deep white matter. The FLAIR and T1-weighted image showed focal, rounded, high-signal intensity lesions on both parts of the globus pallidus (Fig. 2). Single-photon emission computed tomography (SPECT) of the brain showed significant hypoperfusion in both basal ganglia (especially globus pallidus), frontal and temporal lobes at admission (Fig. 3). With a diagnosis of HE, the patient was treated with a high dose of steroid (methylprednisolone 1 g/day) for 7 days. Subsequently, she took steroid pills for an additional 4 weeks, while gradually decreasing the dosage. After 4-5 days of steroid treatment, her bradykinesia improved and she spoke more. After 4 months, her disorientation, cognition level, and physical function improved to a level almost similar to her previous level. A follow-up brain MRI, taken 15 weeks after initial presentation, showed marked improvement on DWI and FLAIR images, but the lesions in both parts of the globus pallidus lost their high-signal intensities, and changed into cyst like lesions (Fig. 2). In addition, the follow-up blood test confirmed normal levels of anti-Tg antibody (60.65 IU/mL) and anti-TPO antibody (18.97 IU/mL).
HE patients demonstrate various neuropsychological symptoms as well as some altered cognitive functions. These symptoms can be classified into two categories according to their occurrence and progression pattern. The first is the vasculitis form, which is similar to stroke and demonstrates accompanying hemiparesis, aphasia, and ataxia; this form may also result in mild cognitive dysfunction. The second category is the diffuse form that is accompanied by relatively slowly progressing cognitive disorders, changes in consciousness, hallucinations, and psychological symptoms [1, 5]. In the case of our patient, the symptoms occurred suddenly, suggestive of the vasculitis form.
The pathophysiological mechanisms of HE have been summarized by two theories [1]. The first theory involves an antineuronal antibody-mediated reaction where the central nervous system (CNS) is subject to an autoimmune reaction that impacts the thyroid and CNS tissues. One study reported that the anti-TPO antibody combined with cerebellar astrocytes in the cerebellum of HE patients but not in Hashimoto thyroiditis patients. Another study reported that anti-TPO antibodies, anti-Tg antibodies, and circulating immune complexes were elevated in the CSF of HE patients [2]. However, 10-20% of healthy people and patients with other types of autoimmune diseases can also test positive for anti-thyroid antibodies [2], which means that these antibodies are likely not specific to HE. Autoimmune vasculitis is another proposed pathomechanism that was developed due to the observed lymphocytic infiltration around blood vessels and into blood vessel walls. Brain SPECT has also shown local or general hypoperfusion, and anti-α-enolase antibodies located mostly inside vascular endothelial cells in HE patients [1].
Although increased anti-thyroid antibodies in the blood or CSF can be of significant diagnostic value for HE, they are not unique and may increase without disease or may increase in patients with different autoimmune diseases. Anti-TPO antibodies combine with microsomes from thyroid cells, a condition for which most HE patients are positive, and anti-Tg antibodies combine with thyroglobulin (required for producing thyroid hormone), which is also often increased in HE patients [1, 2]. Other antibodies, such as the anti-α-enolase antibody, are known to be somewhat more unique to HE. Anti-α-enolase antibodies are distributed inside vascular endothelial cells, explaining why they are considered supportive evidence for vasculitic symptoms of HE [1]. In our case, only the anti-Tg antibody increased to abnormal levels, but recovered after steroid treatment. This observation supports the idea that normalization of the anti-Tg antibody titer can be related to improvement in the patients' condition [6]. Moreover, there was a comment that anti-TPO antibodies were positive in almost HE cases [1], but there was no large study about incidences of anti-TPO & anti-Tg antibody in HE cases. Recent studies reported 27 patients with HE that 23 patients (85%) showed high titer of both antibodies, 2 patients (7.5%) showed high titer of anti-TPO antibody, and 2 patients (7.5%) showed only high titer of anti-Tg antibody [2, 6, 7]. MRI, SPECT, and positron emission tomography are commonly used to evaluate brain disorders in HE patients. MRI shows variable findings, with 50% of patients appearing to be normal, while others show nonspecific findings, such as ischemic changes, demyelination, vasogenic edema, or atrophy [1, 4]. Among these findings, bilateral and focal or diffuse high-signal intensity changes in the subcortical area are the most common [1, 5, 8]. In addition, MRIs show different findings over time, and can show improvement after steroid treatment [3, 4, 8]. Our patient showed widespread diffuse hyperintense lesions on the deep white matter in DWI, T2-weighted, and FLAIR images, and those disappeared in concert with clinical improvement after corticosteroid treatment, but the lesions in both globus pallidus lost their high-signal intensities, and changed into cyst like lesions. Brain SPECT showed significant hypoperfusion in both basal ganglia, frontal and temporal lobes at admission. Forchetti et al. [9] reported global hypoperfusion on brain SPECT in HE patients, and it could be a pathophysiology of HE by autoimmune cerebral vasculitis.
We suggest that the marked improvement of the diffuse hyperintense lesions in DWIs reflect mixed cytotoxic/vasogenic edema resulting from a temporary ischemic injury in the brain tissue associated with brain hypoperfusion. Moreover, the reason for the remnant lesions of high-signal intensity and subsequent change to a cystic form may be the permanent neuronal damage associated with significant hypoperfusion on brain SPECT in both areas of globus pallidus caused by autoimmune reactivity [9].
The current case emphasizes the importance of including DWI in the imaging evaluation of patients with HE in order to assess the possible mechanism of vasculitic changes and to provide another measure of response to steroid therapy. Our case illustrates the peculiar changes in the MR findings, especially in DWI, with hypoperfusion on brain SPECT in patients with HE and allows for a greater understanding of the pathophysiology of HE.
Figures and Tables
Fig. 1
Cone biopsy of thyroid. Infiltration of lymphoid foillicle (A) and Hurthle cell change of surrounding follicular epithelial cells (B).
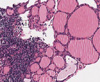
Fig. 2
Initial and followup brain MRI findings. Initial diffusionweighted, FLAIR, T2weighted, T1weighted and contrast images (A and B) showing illdefined diffuse high signal intensity lesions on the deep white matter and globus pallidus. After 3 months (C and D), DWI, FLAIR images show the extent of the lesions, which are markedly decreased compared to the previous MRI and cystic changes occur in bilateral globus pallidus.
References
2. Ferracci F, Moretto G, Candeago RM, Cimini N, Conte F, Gentile M, et al. Anti-thyroid antibodies in the CSF: their role in the pathogenesis of Hashimoto's encephalopathy. Neurology. 2003. 60:712–714.

3. Grommes C, Griffin C, Downes KA, Lerner AJ. Steroid-responsive encephalopathy associated with autoimmune thyroiditis presenting with diffusion MR imaging changes. AJNR Am J Neuroradiol. 2008. 29:1550–1551.

4. Chen C, Qin W, Wei C, Wang X, Li K. Time course of Hashimoto's encephalopathy revealed by MRI: Report of two cases. J Neurol Sci. 2011. 300:169–172.

5. Mijajlovic M, Mirkovic M, Dackovic J, Zidverc-Trajkovic J, Sternic N. Clinical manifestations, diagnostic criteria and therapy of Hashimoto's encephalopathy: report of two cases. J Neurol Sci. 2010. 288:194–196.

6. Blanchin S, Coffin C, Viader F, Ruf J, Carayon P, Potier F, et al. Anti-thyroperoxidase antibodies from patients with Hashimoto's encephalopathy bind to cerebellar astrocytes. J Neuroimmunol. 2007. 192:13–20.
7. Kothbauer-Margreiter I, Sturzenegger M, Komor J, Baumgartner R, Hess CW. Encephalopathy associated with Hashimoto thyroiditis: diagnosis and treatment. J Neurol. 1996. 243:585–593.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download