Abstract
Currently, the increasing numbers of vaccine administrations are associated with increased reports of adverse vaccine reactions. Whilst the general adverse reactions including allergic reactions caused by the vaccine itself or the vaccine components, are rare, they can in some circumstances be serious and even fatal. In accordance with many IgE-mediated reactions and immediate-type allergic reactions, the primary allergens are proteins. The proteins most often implicated in vaccine allergies are egg and gelatin, with perhaps rare reactions to yeast or latex. Numerous studies have demonstrated that the injectable influenza vaccine can be safely administered, although with appropriate precautions, to patients with severe egg allergy, as the current influenza vaccines contain small trace amounts of egg protein. If an allergy is suspected, an accurate examination followed by algorithms is vital for correct diagnosis, treatment and decision regarding re-vaccination in patients with immediate-type reactions to vaccines. Facilities and health care professionals should be available to treat immediate hypersensitivity reactions (anaphylaxis) in all settings where vaccines are administered.
Similar to other drugs, vaccines have the potential to cause allergic reactions [1-3]. Vaccines, specifically individual components of the vaccine, are known to, although rarely, cause serious complications. Even after an allergic reaction after vaccination, it is difficult to ascertain whether the allergic reaction was caused by the vaccine itself or other factors. Recently, mild allergic reactions caused by vaccinations have become common in practice due to an increased amount of vaccinations, however, these mild allergic reactions can still lead to serious complications and therefore require attention.
The vaccine components include active immunizing antigens, conjugating agents, preservatives, stabilizers, antimicrobial agents, adjuvants and culture media used in the preparation of the vaccine, as well as inadvertent contaminants that are introduced during vaccine handling (Table 1). Almost all the vaccine components can be considered as potential triggers of an allergic reaction. Of particular importance are culture derived proteins from egg, gelatin and yeast. Other sources of allergic reaction are antibiotics and vaccination antigens.
The immune-mediated reactions caused by individual components of the vaccines are outlined in Table 2. Allergic responses caused by vaccines are generally of Type I and IV hypersensitivity reactions.
The most immediate reactions are Type I hypersensitivity reactions that are mediated by the interaction of IgE antibodies against a particular vaccine component. These reactions typically occur within minutes of exposure to the relevant allergen and almost always occur within 4 hours of exposure to the relevant allergen, however, possible exceptions for delayed-onset reactions do occur [4]. The most common symptoms of IgE-mediated allergic reactions are urticaria and angioedema, with less common symptoms including nasal congestion, cough, stridor, wheezing, shortness of breath, vomiting, abdominal pain, diarrhea and hypotension. Anaphylaxis, an acute hypersensitivity reaction with multi-organ system involvement can present as a severe life-threatening reaction, or can occur after vaccination.
It has been reported that the average rate for immediate type reactions in children and adolescents is 0.22 per 100,000 doses of vaccinations [2]. A total of 31% of these patients reported immediate type reactions after the first vaccination. This observation suggests either a pre-sensitization to a component of the vaccine or a non-immunologically mediated reaction [1]. In contrast, according to Bohlke et al. [3], the reported cases of potential anaphylaxis after vaccination amount to 0.065 per 100,000 given doses of vaccines.
Type IV hypersensitivity delayed reactions have also been reported, however, these reactions are generally considered to be harmless. Type IV hypersensitivity reactions generally begin 48 hours after vaccination and peak between 72 and 96 hours [5]. These reactions are typically observed following vaccines containing thimerosal, aluminum and anti-microbial agents. The occurrence of such an event is not a contraindication for further vaccinations. Type IV reactions are becoming less frequent as mercury is being removed from modern vaccines. Another reported hypersensitivity reaction includes erythema multiforme. This reaction can be quite severe in children and is triggered by a number of allergens, including vaccine components [5].
The majority of delayed reactions are classified as Type III hypersensitivity and are attributed primarily to the formation of immune complexes, however, less well-defined mechanisms, including T cell-mediated processes, may also play a role [4]. The most common signs of delayed-type reactions include rashes, which may include as urticaria, erythema multiforme, and/or maculopapular eruptions. Angioedema may also occur, paritcularly in association with urticaria or erythema multiforme eruptions. Although uncommon, arthralgia, arthritis, joint swelling, serum sickness, and Henoch-Schönlein purpura may occur, in conjunction with a variety of other hematologic, renal and gastrointestinal manifestations. Some delayed reactions, however, may not be immunologically mediated. Persistent hard nodules at the injection site may involve irritant reactions, usually induced by adjuvants such as aluminum and do not necessarily reflect immunologic hypersensitivity to vaccine constituents.
Rarely, hyperimmunized patients by previous injections of a vaccine (e.g., tetanus vaccination) developed a local immune complex mediated by a Arthus-type reaction at the site of vaccine injection [6,7]. T-cell mediated reactions usually manifest in the form of local eczema, starting from 2-8 hours up to 2 days after vaccination. Sometimes the reaction may extend beyond the injection area and may even become generalized [8,9].
Idiopathic and autoimmune responses are other immune related reactions. Self-reactive antibodies, created by molecular mimicry between the vaccine antigen and endogenous epitope, may be induced by vaccination. For example, idiopathic thrombocytopenic purpura may be produced by several viral infections that may induce autoantibodies to platelet surface glycoprotein [5]. Such reported cases are 1 in 3,000 for rubella virus, 1 in 30,000 for measles, mumps, and rubella (MMR) vaccine and 1 in 6,000 for the measles virus [10]. The Guillain-Barré syndrome (GBS) outbreak in 1976-1977, as a result of a swine influenza vaccine campaign, is the most well documented example of an autoimmune reaction to vaccinations. Briefly, in fear of an influenza pandemic, an 'immunization campaign' took place throughout 1976-1977. Many people immunized with the swine influenza vaccine during the campaign period (approximately 0.04 per 100,000 vaccinations) developed GBS within 6 weeks following immunization. At the end of the 'immunization campaign', however, there were no further reported cases of GBS [11]. Recently the estimated rate of influenza vaccination-related GBS in Korea was reported to be 0-0.025 per 100,000 distributed doses which is considerably lower than 0.04 to less than one case per 100,000 vaccinations reported in previous studies [11-13]. Despite strong epidemiological data of an association between swine flu vaccination and GBS, the biological mechanisms remain to be demonstrated and thus require further research.
A list of vaccine components that can potentially cause allergic reactions are continually updated and are accessible on the website of the Institute for Vaccine Safety: http://www.vaccinesafety.edu. Below is a brief review of the vaccine components that can potentially result in allergic reactions.
In order to protect vaccines from unfavorable conditions such as excessive heat or the freeze-drying process, stabilizers such as sugar (sucrose and lactose), amino acids (monosodium salt of glutamic acid or glycine) and protein (gelatin or human serum albumin) are added. Gelatin is commonly found in every day food, pharmaceuticals, photography, cosmetics, etc. When used in vaccines, gelatin is extensively cross-reactive and is of bovine or porcine origin [14-16]. Gelatin is used as a stabilizer in attenuated viral-containing vaccines such as Japanese encephalitis virus, varicella and MMR (Table 3) and causes the most allergic reactions compared with other vaccine components [17]. In Japan, after the introduction of vaccines not containing gelatin, the incidence of allergic reactions sharply declined [18,19].
Patients allergic to vaccines containing gelatin should be checked for IgE antibody to gelatin using a serum specific IgE test to gelatin (commercially available) or a skin prick test extract which is not yet approved by the Food and Drug Administration (FDA). People with a food allergy to gelatin may develop an anaphylactic reaction following injection with a vaccine containing gelatin, however, it has been reported that people without a gelatin food allergy may also develop an anaphylactic reaction in response to a vaccine containing gelatin [15]. This can be explained by the differing routes of vaccine administration, that is, ingestion versus injection.
If a patient has a history of Type I immediate allergic reaction to gelatin and this allergic reaction is confirmed by a skin test or a serum specific IgE test, a skin test should be performed using specifically the gelatin containing vaccine [4, 17]. If the result is positive, the vaccine should be injected in graded doses under observation (Table 4). If the result is negative, the injection should be carried out normally with observation of the patient continued for another 30 minutes after injection.
To prevent the growth of microorganisms, preservatives such as 2-phenoxyethanol and thimerosal are often added to vaccines. Thimerosal is a neurotoxic organic mercurial compound (50% mercury by weight), however, it has been reported that low concentrations of thimerosal in vaccines does not result in any adverse reactions [20]. Nonetheless, the U.S. Public Health Service and the American Academy of Pediatrics (AAP) urged the removal of thimerosal in vaccines routinely used for infants by the year 1999. Consequently, with the exception of some vaccines, thimerosal has been completely removed or reduced to trace amounts in vaccines for children.
In general, the majority of patients hypersensitive to thimerosal do not experience any adverse reactions to vaccines containing and consequently the administration of vaccines containing thimerosal remains non contraindicative to future vaccination.
To avoid contamination during the manufacturing process, several vaccines include trace amounts of neomycin, streptomycin, and/or polymyxin B.
Currently, only limited reports of allergic reactions due to vaccines containing antibiotics exist. Some patients may experience a Type IV delayed type allergic reaction such as injection site papule 48 to 96 hours following vaccination [21]. Patients who experience an anaphylactic reaction to a specific kind of antibiotic, however, should avoid vaccines containing that specific antibiotic.
Hepatitis B vaccines and human papilloma virus vaccines are created using antigens from the culture of recombinant Saccharomyces cerevisiae (bakers' yeast), thus the vaccines consist of a small amount of yeast protein (up to 5%) [22].
Allergic reactions as a result of the presence of yeast protein in vaccines appear to be extremely rare. After reviewing 180,000 allergic reports in the Vaccine Adverse Event Reporting System, only 15 appeared to result from a reaction to yeast proteins in the vaccines. Furthermore, these 15 cases may have resulted from other variables rather than the presence of yeast protein in the vaccine alone [23]. If a patient is suspected of yeast protein allergy, an IgE antibody to yeast should be measured. Commercially available skin test reagents and skin tests are available and should be performed. If the result is positive, the vaccine should be injected in graded doses under constant observation. If the results of the tests are negative, the vaccinations should be carried out normally and observation of the patient continued for another 30 minutes following vaccination (Table 4).
Latex, used to create a natural rubber latex and dry natural rubber, contains naturally occurring impurities. Such impurities are often responsible for recipient allergic reactions. In contrast, synthetic latex does not contain such impurities and therefore should be considered as an alternative when administering vaccinations. Contact type allergy is the most common type of latex sensitivity [26]. Several cases of latex anaphylaxis have been reported during operations [27,28]. Injection related latex allergies have been reported, however such allergies rarely occur after vaccination. Only one anaphylactic reaction after vaccination has been reported [29].
MMR vaccines are created by cell cultures using a chicken embryo fibroblast. These vaccines contain minimal to negligible amounts of egg protein. Consequently, even in children with mild or severe egg allergies, MMR vaccinations result in a low risk of anaphylaxis [30,31].
Mild adverse reactions to MMR may occur regardless of skin test results in children with egg allergies. MMR skin tests do not predict an allergic reaction [32,33]. Interestingly, the majority of other hypersensitivity reactions following MMR vaccination are caused by components other than egg protein.
The influenza vaccine is grown in an allantoic fluid in embryonated chicken eggs. The vaccine contains measurable quantities of egg proteins that vary broadly from 0.2 to 42 µg/mL [34]. In theory, the influenza vaccine may result in systemic reactions when injected into egg-allergic patients. The safe administration of other vaccines containing egg protein has been investigated. Egg-allergic patients administered with either several graded doses of the influenza vaccine [35], or a two-dose protocol with the influenza vaccine presented with no negative vaccine reactions [36]. In the former study (several graded doses of the influenza vaccine), the egg content of the vaccine was not stated; in the latter study (a two-dose protocol with the influenza vaccine), the egg content was less than or equal to 1.2 µg/mL [36]. When the egg content of the vaccine could not be determined, it appeared that the appropriate clinical tests are the prick (full strength) and intradermal (1:100) skin tests for the appropriate vaccine. If the skin test results are positive, a more cautious (several dose) protocol for vaccine administration must be administered [34].
Recently, 28 studies have reported that more than 4,300 egg-allergic individuals who received influenza vaccines presented with no serious reactions (including respiratory distress or hypotension) and that only a low rate percentage of patients presented with minor reactions (hives and mild wheezing). Such results did not significantly differ from the rate in non-egg-allergic controls [17,37,38]. The majority of these studies specifically included patients with histories of severe anaphylaxis (n=656) as a result of egg ingestion. These patients did not experience hypersensitivity due to trace amounts of egg protein.
In 2011, the United States Centers for Disease Control (USCDC) reported that the amount of ovalbumin in an influenza vaccine circulated in the US was less than 1 µg per 0.5 mL vaccination dose since 2011 [39,40]. The USCDC reported that all influenza vaccines distributed in the US contains low amounts of ovalbumin.
Both the Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices and the American Academy of Pediatrics' Committee on Infectious Diseases have recently concluded that egg allergy of any severity (including anaphylaxis) is not a contraindication to the administration of further influenza vaccine, but rather a precaution (Fig. 1) [33,40].
Whilst the intranasally administration of live attenuated influenza vaccine (LAIV) contains a low amount of ovalbumin, the majority of published studies support the injectable trivalent inactivated vaccine (TIV), and thus support TIV rather than LAIV for egg-allergic recipients.
From 2004 to 2006, 9 different influenza vaccines were distributed in Korea by either domestic and/or foreign companies. These 9 different influenza vaccines were measured for albumin content. Each of the 9 different influenza vaccines were below 1.2 µg per 1 mL dose of albumin content (Fig. 2) [41]. The domestic vaccination guideline, however, did not list the amount of egg protein in all influenza vaccines being circulated in Korea.
The risk of not vaccinating remains a greater risk than that of vaccinating. Patients who report that they are egg allergic should be referred to an allergist, who can assess the current status of the patient's egg allergy as previously diagnosed by history and skin tests or blood tests for IgE antibody to egg. Importantly, this should not delay influenza vaccination [17]. Skin testing of egg-allergic persons with the influenza vaccine before administration is not recommended due to low sensitivity of the test and specificity in predicting serious reactions to vaccine administration. Dividing the dose of vaccine is also not required because even the most severely egg-allergic patients can tolerate the full vaccine dose without reaction [33,39,40].
The primary precaution of adverse influenza vaccination to those who are egg allergic, include: that the vaccine be administered in a clinical setting whereby anaphylaxis can be recognized and immediately treated should it occur, and that patients should remain under observation for at least 30 minutes after vaccination. The current guidelines indicate that egg-allergic patients with a history of hives only after egg ingestion, can receive influenza vaccine in a primary care physician's office provided that the appropriate personnel and equipment are available. Patients with a history of more severe reactions to egg ingestion, however, should receive their vaccine in an allergist's office [39,40].
Egg protein containing vaccination candidates are routinely asked if they are allergic to soft-bolied eggs such as "scrambled eggs" [39,40]. The influenza vaccine is not heated during manufacturing and therefore still contains egg proteins that would otherwise be destroyed by heat. It should be noted, however, that a patient has shown to be allergic to a heat-labile egg protein [42]. Thus, it may be difficult to identify all persons allergic to egg proteins present in influenza or other vaccines purely by patient clinical history.
Whilst the Yellow fever vaccine is grown in chicken embryos and contains residual egg protein, the amount of residual egg protein remains unavailable to the manufacturer. Patients with allergic reactions to egg-containing vaccines should be evaluated for IgE antibody to eggs. There are commercially available skin test reagents and serum specific IgE tests to eggs. Individuals who react to egg ingestion should be evaluated prior to administration of yellow fever vaccine. If the history is consistent with an immediate-type allergic reaction to egg and is confirmed by skin tests or serum specific IgE, skin testing should be performed with yellow fever vaccine before administration [4,17]. If the result is positive, the vaccine should be injected in graded doses under observation. If negative, the injection should be carried out normally with close observation for another 30 minutes following vaccination.
Patients with suspected allergies to vaccines or vaccine components should be evaluated by an allergist. A meticulous medical history of the patient should be recorded to determine the nature and timing of the reaction to the vaccine in question and vaccine constituents, such as gelatin, egg, latex and yeast.
All suspected anaphylactic reactions to vaccines should ideally be evaluated in an attempt to determine the culprit allergen. It is disturbing that a patient who experiences an IgE-mediated reaction following immunization is often labeled as being "allergic" to the vaccine and is thus advised against receiving future doses without further investigation. This approach should be avoided as it may leave patients inadequately immunized if they unnecessarily avoid the vaccines to which they are not allergic to, or if the vaccine can be administered safely without causing an allergic reaction. In addition, not knowing the particular constituents of a vaccine to which the patient is allergic to may pose a risk to future doses of other vaccines that contain similar constituents.
The approach to a patient who may be allergic to eggs and require the influenza vaccine should be distinguished from the approach to a patient who has had an apparent allergic reaction to the influenza vaccination. Domestic vaccination guidelines in Korea should list the amount of egg protein in all influenza vaccines to provide further information about vaccines being administered domestically.
Figures and Tables
Fig. 1
Recommendations regarding influenza vaccination for persons who report allergy to eggs according to the Advisory Committee on Immunization Practices, United States, 2012-13 influenza season. TIV, trivalent inactivated vaccine. a)Persons with egg allergy may tolerate egg in baked products (for example bread or cake). Tolerance to egg-containing foods does not exclude the possibility of egg allergy. Adapted from Centers for Disease Control and Prevention [40].

Fig. 2
The range of ovalbumin content in the different manufacturing companies (A-I) in Korea. Adapted with Roh et al. [41], with permission from The Korean Academy of Pediatric Allergy and Respiratory Disease.

Table 2
Synopsis of potential immune-mediated reactions to vaccines
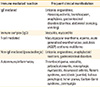
Adapted from Fritsche et al. [1].
Table 3
Gelatin containing vaccine and its contents

Adapted from Kelso et al. [17], with permission from Elsevier Ltd.
Table 4
Administration of a vaccine in graded doses to patients with positive skin test

Adapted from Kelso et al. [17], with permission from Elsevier Ltd.
Vaccine where the full dose was 0.5 mL, physicians should give the following doses at 15-20 minute intervals. Patients under observation if an anaphylactic reaction should it occur. Patients were observed for at least 30 minutes after the last dose.
References
1. Fritsche PJ, Helbling A, Ballmer-Weber BK. Vaccine hypersensitivity: update and overview. Swiss Med Wkly. 2010; 140:238–246.
2. Wood RA, Berger M, Dreskin SC, et al. An algorithm for treatment of patients with hypersensitivity reactions after vaccines. Pediatrics. 2008; 122:e771–e777.

3. Zent O, Arras-Reiter C, Broeker M, Hennig R. Immediate allergic reactions after vaccinations: a post-marketing surveillance review. Eur J Pediatr. 2002; 161:21–25.

4. Bohlke K, Davis RL, Marcy SM, et al. Risk of anaphylaxis after vaccination of children and adolescents. Pediatrics. 2003; 112:815–820.

5. Siegrist CA. Mechanisms underlying adverse reactions to vaccines. J Comp Pathol. 2007; 137:Suppl 1. S46–S50.

7. Facktor MA, Bernstein RA, Firemann P. Hypersensitivity to tetanus toxoid. J Allergy Clin Immunol. 1973; 52:1–12.

8. Cox NH, Moss C, Forsyth A. Allergy to non-toxoid constituents of vaccines and implications for patch testing. Contact Dermatitis. 1988; 18:143–146.

9. Vogt T, Landthaler M, Stolz W. Generalized eczema in an 18-month-old boy due to phenoxyethanol in DPT vaccine. Contact Dermatitis. 1998; 38:50–51.

10. Miller E, Waight P, Farrington CP, Andrews N, Stowe J, Taylor B. Idiopathic thrombocytopenic purpura and MMR vaccine. Arch Dis Child. 2001; 84:227–229.
11. Haber P, Sejvar J, Mikaeloff Y, DeStefano F. Vaccines and Guillain-Barré syndrome. Drug Saf. 2009; 32:309–323.

12. Choe YJ, Cho H, Kim SN, Bae GR, Lee JK. Serious adverse events following receipt of trivalent inactivated influenza vaccine in Korea, 2003-2010. Vaccine. 2011; 29:7727–7732.

13. Liang XF, Li L, Liu DW, et al. Safety of influenza A (H1N1) vaccine in postmarketing surveillance in China. N Engl J Med. 2011; 364:638–647.

14. Kelso JM, Jones RT, Yunginger JW. Anaphylaxis to measles, mumps, and rubella vaccine mediated by IgE to gelatin. J Allergy Clin Immunol. 1993; 91:867–872.

15. Sakaguchi M, Nakayama T, Inouye S. Food allergy to gelatin in children with systemic immediate-type reactions, including anaphylaxis, to vaccines. J Allergy Clin Immunol. 1996; 98(6 Pt 1):1058–1061.

16. Bogdanovic J, Halsey NA, Wood RA, Hamilton RG. Bovine and porcine gelatin sensitivity in children sensitized to milk and meat. J Allergy Clin Immunol. 2009; 124:1108–1110.

17. Kelso JM, Greenhawt MJ, Li JT, et al. Adverse reactions to vaccines practice parameter 2012 update. J Allergy Clin Immunol. 2012; 130:25–43.

18. Nakayama T, Aizawa C. Change in gelatin content of vaccines associated with reduction in reports of allergic reactions. J Allergy Clin Immunol. 2000; 106:591–592.

19. Kuno-Sakai H, Kimura M. Removal of gelatin from live vaccines and DTaP: an ultimate solution for vaccine-related gelatin allergy. Biologicals. 2003; 31:245–249.

20. Centers for Disease Control and Prevention (CDC). Thimerosal in vaccines: a joint statement of the American Academy of Pediatrics and the Public Health Service. MMWR Morb Mortal Wkly Rep. 1999; 48:563–565.
22. Grabenstein J. Clinical management of hypersensitivities to vaccine components. Hosp Pharm. 1997; 32:77–87.
23. DiMiceli L, Pool V, Kelso JM, Shadomy SV, Iskander J. V.A.E.R.S. Team. Vaccination of yeast sensitive individuals: review of safety data in the US vaccine adverse event reporting system (VAERS). Vaccine. 2006; 24:703–707.

24. Kaaber K, Nielsen AO, Veien NK. Vaccination granulomas and aluminium allergy: course and prognostic factors. Contact Dermatitis. 1992; 26:304–306.

25. Bergfors E, Trollfors B, Inerot A. Unexpectedly high incidence of persistent itching nodules and delayed hypersensitivity to aluminium in children after the use of adsorbed vaccines from a single manufacturer. Vaccine. 2003; 22:64–69.

27. Kim KW, Kim SM, Ko EM, et al. Latex anaphylaxis during labor: case report. Korean J Obstet Gynecol. 2002; 45:311–314.
28. Lee W, Lee JH, Park DJ, Kim HH. A case of anaphylactic shock attributed to latex allergy during gastric cancer surgery. J Korean Surg Soc. 2011; 81:Suppl 1. S30–S33.

30. James JM, Burks AW, Roberson PK, Sampson HA. Safe administration of the measles vaccine to children allergic to eggs. N Engl J Med. 1995; 332:1262–1266.

31. Baxter DN. Measles immunization in children with a history of egg allergy. Vaccine. 1996; 14:131–134.

32. Nam SY, Jung EH, Jung JA, et al. Measles, mumps, and rubella immunization in children with egg allergies. J Korean Pediatr Soc. 2001; 44:1031–1035.
33. Pickering LK, Baker CJ, Kimberlin DW, Long SS. Red Book: 2012 report of the Committee on Infectious Diseases. Elk Grove Village: American Academy of Pediatrics;2012.
34. Zeiger RS. Current issues with influenza vaccination in egg allergy. J Allergy Clin Immunol. 2002; 110:834–840.

35. Murphy KR, Strunk RC. Safe administration of influenza vaccine in asthmatic children hypersensitive to egg proteins. J Pediatr. 1985; 106:931–933.

36. James JM, Zeiger RS, Lester MR, et al. Safe administration of influenza vaccine to patients with egg allergy. J Pediatr. 1998; 133:624–628.

37. Des Roches A, Paradis L, Gagnon R. Egg-allergic patients can be safely vaccinated against influenza. J Allergy Clin Immunol. 2012; 130:1213–1216.

38. Greenhawt MJ, Spergel JM, Rank MA, et al. Safe administration of the seasonal trivalent influenza vaccine to children with severe egg allergy. Ann Allergy Asthma Immunol. 2012; 109:426–430.

39. National Center for Immunization and Respiratory Diseases. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011; 60:1–64.
40. Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP): United States, 2012-13 influenza season. MMWR Morb Mortal Wkly Rep. 2012; 61:613–618.
41. Roh EJ, Chung EH, Kim JK. Quantitative analysis of egg protein by ELISA in distributed influenza vaccine in Korea. Pediatr Allergy Respir Dis. 2009; 19:345–353.
42. Kelso JM. Raw egg allergy: a potential issue in vaccine allergy. J Allergy Clin Immunol. 2000; 106:990.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download