Abstract
Production of thymus-dependent antibodies by autoreactive B cells requires help from T cells. Follicular helper T (Tfh) cells are a unique lineage of CD4+ T subsets present in the follicles of peripheral lymphoid tissues which functions primarily to provide help to cognate B cells. Within germinal centers Tfh cells stimulate germinal center B cells to undergo affinity maturation, Ig class switching, and differentiation to memory B cells and plasma cells. Proposals that activity of Tfh cells is crucial for long-lived humoral autoimmunity are supported by the correlation of numbers and/or functions of Tfh cells with disease activity in many autoimmune disorders. In this review, we discuss recent findings regarding Tfh cell development and function. In addition, we discuss putative roles of Tfh cells in the pathogenesis and highlight the potential of Tfh cells as therapeutic targets in autoimmune diseases.
Figures and Tables
Fig. 1
Pathways of TD autoantibody production. Autoreactive T and B cells develop in the thymus and bone marrow (BM) and populate in the peripheral lymphoid organs, such as spleen and lymph nodes (LN) (1). In these sites, autoantigen-pulsed dendritic cells license CD4+ T cells to differentiate to extrafollicular helper T (Tefh) cells, which in turn activate their cognate B cells to differentiate to antibody-secreting cells (2). In addition to this extra-follicular pathway, more intensive activation and maturation of B cells take place in the germinal center (GC) within the follicle (3). As a result of the GC reaction, affinity matured and isotype switched memory B cells and plasma cells plasma cells (PC) develop, and exit to extra-follicular area (4). Some plasma cells migrate to bone marrow in search of survival niche (5). Autoantibodies infiltrate to target tissues and trigger inflammatory cascades, finally leading to tissue damage (6).
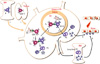
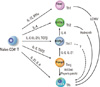
Fig. 2
Development and plasticity of Th subsets. Naïve CD4+ T cells differentiate to distinct subsets of effector cells upon priming with antigens. The cytokine milieu during priming is important for selective expression of lineage-specific master transcription factors. Each subset is prone to be converted to others under certain conditions. Th17 conversion to Tfh is speculative to date, so indicated as a broken line.
ACKNOWLEDGEMENTS
This work was supported by a National Research Foundation grant funded by the Korean Government (MEST; 2009-0081790).
References
1. Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000. 192:1553–1562.

2. Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000. 192:1545–1552.

3. Odegard JM, Marks BR, DiPlacido LD, Poholek AC, Kono DH, Dong C, et al. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J Exp Med. 2008. 205:2873–2886.

4. King C. New insights into the differentiation and function of T follicular helper cells. Nat Rev Immunol. 2009. 9:757–766.

5. Hiepe F, Dorner T, Hauser AE, Hoyer BF, Mei H, Radbruch A. Long-lived autoreactive plasma cells drive persistent autoimmune inflammation. Nat Rev Rheumatol. 2011. 7:170–178.

6. Craft JE. Follicular helper T cells in immunity and systemic autoimmunity. Nat Rev Rheumatol. 2012. 8:337–347.

7. Ma CS, Deenick EK, Batten M, Tangye SG. The origins, function, and regulation of T follicular helper cells. J Exp Med. 2012. 209:1241–1253.

8. Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol. 2009. 10:167–175.

9. Jang E, Cho SH, Park H, Paik DJ, Kim JM, Youn J. A positive feedback loop of IL-21 signaling provoked by homeostatic CD4+CD25- T cell expansion is essential for the development of arthritis in autoimmune K/BxN mice. J Immunol. 2009. 182:4649–4656.

10. Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008. 29:127–137.

11. Dent AL, Shaffer AL, Yu X, Allman D, Staudt LM. Control of inflammation, cytokine expression, and germinal center formation by BCL-6. Science. 1997. 276:589–592.

12. Fukuda T, Yoshida T, Okada S, Hatano M, Miki T, Ishibashi K, et al. Disruption of the Bcl-6 gene results in an impaired germinal center formation. J Exp Med. 1997. 186:439–448.

13. Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, et al. Bcl-6 and Blimp1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009. 325:1006–1010.

14. Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, et al. Bcl-6 mediates the development of T follicular helper cells. Science. 2009. 325:1001–1005.

15. Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009. 31:457–468.

16. Kusam S, Toney LM, Sato H, Dent AL. Inhibition of Th2 differentiation and GATA-3 expression by BCL-6. J Immunol. 2003. 170:2435–2441.

17. Mondal A, Sawant D, Dent AL. Transcriptional repressor BCL-6 controls Th17 responses by controlling gene expression in both T cells and macrophages. J Immunol. 2010. 184:4123–4132.

18. Fazilleau N, McHeyzer-Williams LJ, Rosen H, McHeyzer-Williams MG. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat Immunol. 2009. 10:375–384.

19. Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010. 207:353–363.

20. Zotos D, Coquet JM, Zhang Y, Light A, D'Costa K, Kallies A, et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. 2010. 207:365–378.

21. Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008. 29:138–149.

22. Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, et al. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS One. 2011. 6:e17739.

23. Harker JA, Lewis GM, Mack L, Zuniga EI. Late interleukin-6 escalates T follicular helper cell responses and controls a chronic viral infection. Science. 2011. 334:825–829.

24. McAdam AJ, Chang TT, Lumelsky AE, Greenfield EA, Boussiotis VA, Duke-Cohan JS, et al. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4+ T cells. J Immunol. 2000. 165:5035–5040.

25. Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol. 2009. 10:385–393.

26. Akiba H, Takeda K, Kojima Y, Usui Y, Harada N, Yamazaki T, et al. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. J Immunol. 2005. 175:2340–2348.

27. Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl-6. Immunity. 2011. 34:932–946.

28. Gigoux M, Shang J, Pak Y, Xu M, Choe J, Mak TW, et al. Inducible costimulator promotes helper T-cell differentiation through phosphoinositide 3-kinase. Proc Natl Acad Sci U S A. 2009. 106:20371–20376.

29. Rolf J, Bell SE, Kovesdi D, Janas ML, Soond DR, Webb LM, et al. Phosphoinositide 3-kinase activity in T cells regulates the magnitude of the germinal center reaction. J Immunol. 2010. 185:4042–4052.

30. Vinuesa CG, Cook MC, Angelucci C, Athanasopoulos V, Rui L, Hill KM, et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005. 435:452–458.

31. Mages HW, Hutloff A, Heuck C, Buchner K, Himmelbauer H, Oliveri F, et al. Molecular cloning and characterization of murine ICOS and identification of B7h as ICOS ligand. Eur J Immunol. 2000. 30:1040–1047.

32. Kroenke MA, Eto D, Locci M, Cho M, Davidson T, Haddad EK, et al. Bcl-6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. J Immunol. 2012. 188:3734–3744.

33. Pot C, Jin H, Awasthi A, Liu SM, Lai CY, Madan R, et al. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J Immunol. 2009. 183:797–801.

34. Hiramatsu Y, Suto A, Kashiwakuma D, Kanari H, Kagami S, Ikeda K, et al. c-Maf activates the promoter and enhancer of the IL-21 gene, and TGF-beta inhibits c-Maf-induced IL-21 production in CD4+ T cells. J Leukoc Biol. 2010. 87:703–712.

35. Schraml BU, Hildner K, Ise W, Lee WL, Smith WA, Solomon B, et al. The AP-1 transcription factor Batf controls T(H)17 differentiation. Nature. 2009. 460:405–409.

36. Betz BC, Jordan-Williams KL, Wang C, Kang SG, Liao J, Logan MR, et al. Batf coordinates multiple aspects of B and T cell function required for normal antibody responses. J Exp Med. 2010. 207:933–942.

37. Ise W, Kohyama M, Schraml BU, Zhang T, Schwer B, Basu U, et al. The transcription factor BATF controls the global regulators of class-switch recombination in both B cells and T cells. Nat Immunol. 2011. 12:536–543.

38. Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature. 2003. 421:282–287.

39. Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008. 455:764–769.

40. Linterman MA, Rigby RJ, Wong RK, Yu D, Brink R, Cannons JL, et al. Follicular helper T cells are required for systemic autoimmunity. J Exp Med. 2009. 206:561–576.

41. Ma CS, Nichols KE, Tangye SG. Regulation of cellular and humoral immune responses by the SLAM and SAP families of molecules. Annu Rev Immunol. 2007. 25:337–379.

42. Cannons JL, Qi H, Lu KT, Dutta M, Gomez-Rodriguez J, Cheng J, et al. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity. 2010. 32:253–265.

43. Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011. 17:975–982.

44. Pelletier N, McHeyzer-Williams LJ, Wong KA, Urich E, Fazilleau N, McHeyzer-Williams MG. Plasma cells negatively regulate the follicular helper T cell program. Nat Immunol. 2010. 11:1110–1118.

45. Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone I Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986. 136:2348–2357.
46. Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009. 30:646–655.

47. Fahey LM, Wilson EB, Elsaesser H, Fistonich CD, McGavern DB, Brooks DG. Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J Exp Med. 2011. 208:987–999.

48. King IL, Mohrs M. IL-4-producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. J Exp Med. 2009. 206:1001–1007.

49. Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, et al. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer's patches. Science. 2009. 323:1488–1492.

50. Bertossi A, Aichinger M, Sansonetti P, Lech M, Neff F, Pal M, et al. Loss of Roquin induces early death and immune deregulation but not autoimmunity. J Exp Med. 2011. 208:1749–1756.

51. Grammer AC, Slota R, Fischer R, Gur H, Girschick H, Yarboro C, et al. Abnormal germinal center reactions in systemic lupus erythematosus demonstrated by blockade of CD154-CD40 interactions. J Clin Invest. 2003. 112:1506–1520.

52. Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011. 34:108–121.

53. Ma J, Zhu C, Ma B, Tian J, Baidoo SE, Mao C, et al. Increased frequency of circulating follicular helper T cells in patients with rheumatoid arthritis. Clin Dev Immunol. 2012. 2012:827480.

54. Simpson N, Gatenby PA, Wilson A, Malik S, Fulcher DA, Tangye SG, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010. 62:234–244.

55. Chang A, Henderson SG, Brandt D, Liu N, Guttikonda R, Hsieh C, et al. In situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. J Immunol. 2011. 186:1849–1860.

56. Weyand CM, Kang YM, Kurtin PJ, Goronzy JJ. The power of the third dimension: tissue architecture and autoimmunity in rheumatoid arthritis. Curr Opin Rheumatol. 2003. 15:259–266.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download