Abstract
Oral food challenge is a definitive diagnostic test for immediate and occasionally delayed adverse reaction to foods. The gold standard for diagnosing food allergy is still the double-blind, placebo-controlled food challenge, but it is time-consuming, expensive and troublesome for physician and patients. Open oral food challenge controlled by trained personnel is useful and sufficient methods when concern of bias is low. We aimed to provide a practical guideline for oral food challenge in children for the diagnosis of suspected food allergy or the evaluation of food tolerance. We considered reasons, types, indications, contraindications, risks, benefits, detailed methods, practical performance, interpretations of test results, and treatments for the adverse reactions of oral food challenge.
References
1. Hong SJ, Ahn KM, Lee SY, Kim KE. The prevalences of asthma and allergic diseases in Korean children. Pediatr Allergy Respir Dis(Korea). 2008; 18:15–25.

2. Chun YH, Yang HJ, Pyun BY, Yum HY, Ahn KM, Lee SY, et el. Sensitizations and clinical symptoms of food allergy in Korean children. Program and abstract, the 60th Annual Fall Meeting of the Korean Pediatric Society; 2010 Oct 22–23; Seoul, Korea. Reston: The Korean Pediatric Society;2010. p. 274.
3. Sicherer SH. Clinical implications of cross-reactive food allergens. J Allergy Clin Immunol. 2001; 108:881–90.

4. Bock SA, Sampson HA, Atkins FM, Zeiger RS, Lehrer S, Sachs M, et al. Double-blind, placebocontrolled food challenge (DBPCFC) as an office procedure: a manual. J Allergy Clin Immunol. 1988; 82:986–97.
5. Rancé F, Deschildre A, Villard-Truc F, Gomez SA, Paty E, Santos C, et al. Oral food challenge in children: an expert review. Eur Ann Allergy Clin Immunol. 2009; 41:35–49.
6. Niggemann B, Beyer K. Diagnosis of food allergy in children: toward a standardization of food challenge. J Pediatr Gastroenterol Nutr. 2007; 45:399–404.

7. Bindslev-Jensen C, Ballmer-Weber BK, Bengtsson U, Blanco C, Ebner C, Hourihane J, et al. Standardization of food challenges in patients with immediate reactions to foods: position paper from the European Academy of Allergology and Clinical Immunology. Allergy. 2004; 59:690–7.
8. Nowak-Wegrzyn A, Assa'ad AH, Bahna SL, Bock SA, Sicherer SH, Teuber SS, et al. Work Group report: oral food challenge testing. J Allergy Clin Immunol. 2009; 123(6 Suppl):S365–83.
9. Sporik R, Hill DJ, Hosking CS. Specificity of allergen skin testing in predicting positive open food challenges to milk, egg and peanut in children. Clin Exp Allergy. 2000; 30:1540–6.

10. Verstege A, Mehl A, Rolinck-Werninghaus C, Staden U, Nocon M, Beyer K, et al. The predictive value of the skin prick test weal size for the outcome of oral food challenges. Clin Exp Allergy. 2005; 35:1220–6.

11. Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001; 107:891–6.

12. Sohn MH, Lee SY, Kim KE. Prediction of buckwheat allergy using specific IgE concentrations in children. Allergy. 2003; 58:1308–10.

14. Niggemann B, Rolinck-Werninghaus C, Mehl A, Binder C, Ziegert M, Beyer K. Controlled oral food challenges in children: when indicated, when superfluous? Allergy. 2005; 60:865–70.
15. Hourihane JO, Grimshaw KE, Lewis SA, Briggs RA, Trewin JB, King RM, et al. Does severity of low-dose, double-blind, placebocontrolled food challenges reflect severity of allergic reactions to peanut in the community? Clin Exp Allergy. 2005; 35:1227–33.

16. Busse PJ, Nowak-Wegrzyn AH, Noone SA, Sampson HA, Sicherer SH. Recurrent peanut allergy. N Engl J Med. 2002; 347:1535–6.

17. Fleischer DM, Conover-Walker MK, Christie L, Burks AW, Wood RA. The natural progression of peanut allergy: Resolution and the possibility of recurrence. J Allergy Clin Immunol. 2003; 112:183–9.

18. Fleischer DM, Conover-Walker MK, Christie L, Burks AW, Wood RA. Peanut allergy: recurrence and its management. J Allergy Clin Immunol. 2004; 114:1195–201.

19. Eigenmann PA, Caubet JC, Zamora SA. Continuing food-avoidance diets after negative food challenges. Pediatr Allergy Immunol. 2006; 17:601–5.

20. David TJ. Anaphylactic shock during elimination diets for severe atopic eczema. Arch Dis Child. 1984; 59:983–6.

21. Flinterman AE, Knulst AC, Meijer Y, Bruijn-zeel-Koomen CA, Pasmans SG. Acute allergic reactions in children with AEDS after prolonged cow's milk elimination diets. Allergy. 2006; 61:370–4.

22. Bernstein IL, Storms WW. Practice parameters for allergy diagnostic testing. Joint Task Force on Practice Parameters for the Diagnosis and Treatment of Asthma. The American Academy of Allergy, Asthma and Immunology and the American College of Allergy, Asthma and Immunology. Ann Allergy Asthma Immunol. 1995; 75(6 Pt 2):543–625.
23. Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000; 161:309–29.
24. Bernstein IL, Li JT, Bernstein DI, Hamilton R, Spector SL, Tan R, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008; 100(3 Suppl 3):S1–148.

25. Hourihane JO'B. Kilburn SA, Nordlee JA, Hefle SL, Taylor SL, Warner JO. An evaluation of the sensitivity of subjects with peanut allergy to very low doses of peanut protein: a randomized, double-blind, placebocontrolled food challenge study. J Allergy Clin Immunol. 1997; 100:596–600.
26. Hansen TK, Bindslev-Jensen C. Codfish allergy in adults. Identification and diagnosis. Allergy. 1992; 47:610–7.

27. Bindslev-Jensen C. Standardization of double-blind, placebocontrolled food challenges. Allergy. 2001; 56(Suppl 67):75–7.

28. Moneret-Vautrin DA. Cow's milk allergy. Allerg Immunol (Paris). 1999; 31:201–10.
29. Wensing M, Penninks AH, Hefle SL, Akkerdaas JH, van Ree R, Koppelman SJ, et al. The range of minimum provoking doses in hazelnut-allergic patients as determined by double-blind, placebocontrolled food challenges. Clin Exp Allergy. 2002; 32:1757–62.

Fig. 1.
Flow sheet for the diagnostic work-up of children with suspected food-related clinical symptoms. IgE, immunoglobulin E; SPT, skin prick test. ∗In cases with milk, egg, peanut, and white fish. (Immunocap, Phadia AB, Uppsala, Sweden)
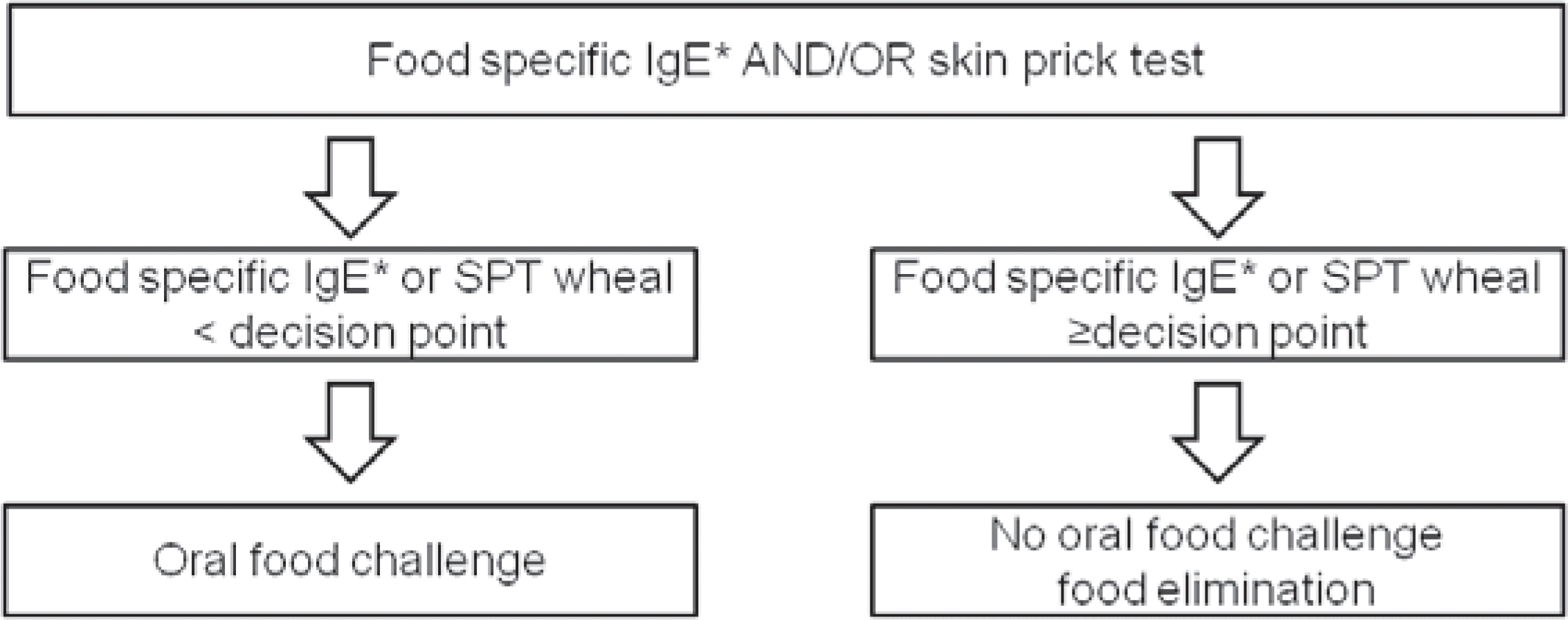
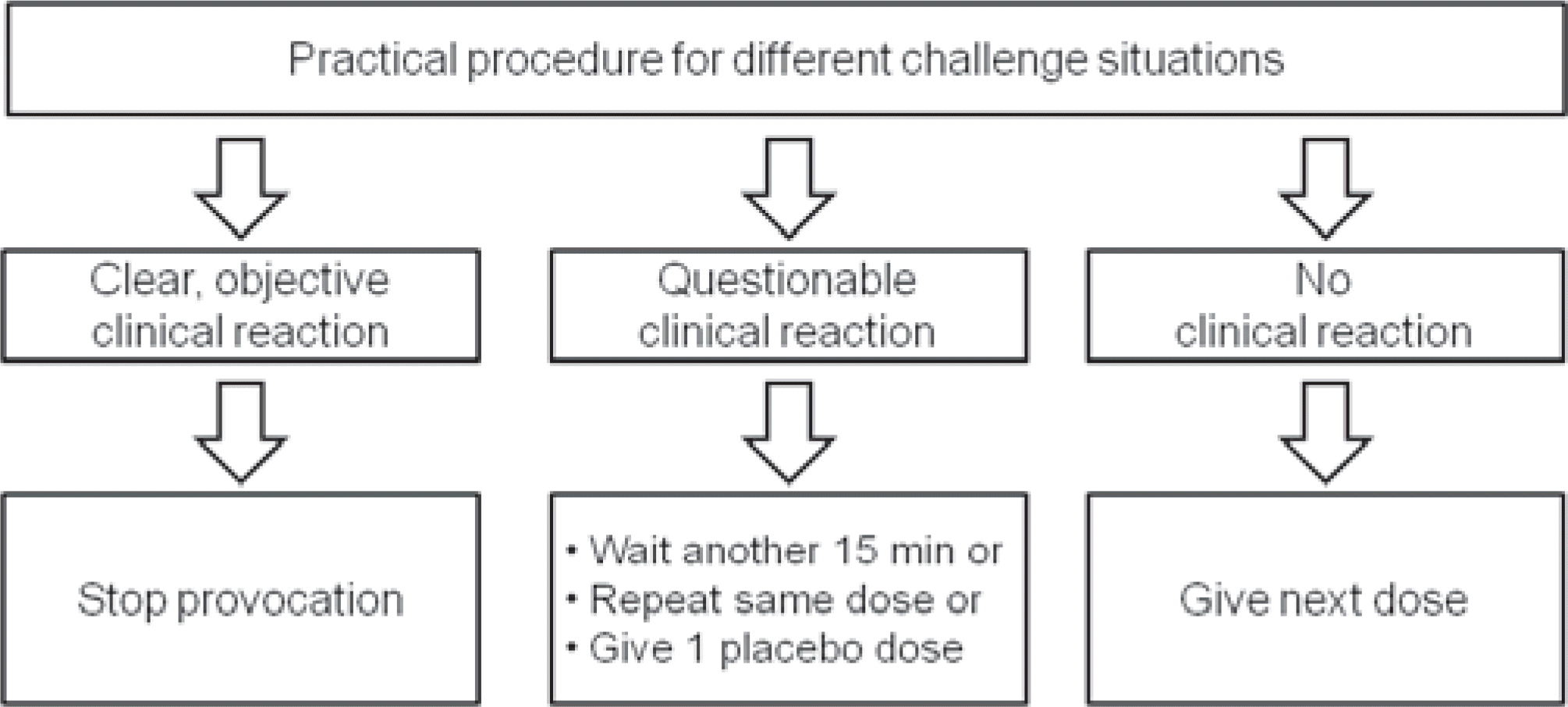
Table 1.
Guidelines for Discontinuation of Medications before Oral Food Challenge (OFC)
Table 2.
Case Examples of Challenge Doses for Oral Food Challenges
| Example 1) Egg∗, boiled, medium-sized (total dose 55 g): preparation with cutting. | |
|---|---|
| Time (min) | Dose† |
| 0 | Lip provocation† |
| 15 | 1/48† |
| 30 | 1/24† |
| 45 | 1/8† |
| 60 | 1/4† |
| 75 | Rest† |
| 135 | (observation) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download