Abstract
Although intravenous recombinant tissue plasminogen activator (IV rt-PA) is effective in many cases of acute ischemic stroke, the neurologic symptoms can worsen after IV rt-PA because of sustained vessel occlusion. For such cases, several reperfusion modalities are available, including intra-arterial thrombolysis (IAT), carotid endarterectomy, and superficial temporal artery-middle cerebral artery (STA-MCA) bypass. Invasive procedures, such as major surgery, should be generally avoided within 24 hours after the administration of IV rt-PA. A 66-year-old man with no previous medical history developed left hemiparesis. A computed tomography scan revealed no acute lesion and he received IV rt-PA within 1.5 hours after symptom onset. Emergent magnetic resonance imaging showed significant diffusion-perfusion mismatch. He received IAT 2 hours after IV rt-PA administration, but IAT failed because of total occlusion of the cervical internal carotid artery. We initially planned to perform STA-MCA bypass the next morning because he had received IV rt-PA, but, 8 hours after IV rt-PA administration, his hemiparesis worsened from motor grade 3/4 to motor grade 1/2. Because of the large perfusion defect in both MCA divisions, double-barrel STA-MCA bypass was performed 10 hours after IV rt-PA administration. His symptoms rapidly improved after surgery and his modified Rankin Scale score 3 months later was grade 0. We suggest that emergent double-barrel bypass can be a viable option in patients who have perfusion defects of both MCA divisions in acute ischemic stroke after IV rt-PA administration.
Superficial temporal artery-middle cerebral artery (STA-MCA) bypass has been generally used for stroke prevention in patients with chronic cerebrovascular insufficiency such as in Moyamoya disease and atherosclerotic occlusion.5)19) A recent randomized controlled trial, the Carotid Occlusion Surgery Study (COSS), concluded that STA-MCA bypass did not provide an overall benefit for stroke prevention in patients with symptomatic atherosclerotic internal carotid artery (ICA) occlusion.14) However, patients in the COSS underwent surgery during the chronic stage, and it remains unknown whether STA-MCA bypass can provide any beneficial effects in the acute stage of ischemic stroke. Emergent STA-MCA bypass is not usually performed for acute ischemic stroke in most hospitals.
Intravenous recombinant tissue plasminogen activator (IV rt-PA) is effective for acute ischemic stroke in many cases, but the clinical symptoms of some patients can worsen after the initiation of IV rt-PA because of sustained vessel occlusion. For such cases, several reperfusion modalities are available, including mechanical thrombectomy, angioplasty and stenting, carotid endarterectomy (CEA), and extracranial to intracranial bypass. Invasive procedures, such as major surgery, should generally be avoided within 24 hours after the administration of IV rt-PA. Here, we report a case in which emergent double-barrel STA-MCA bypass was performed shortly after the administration of IV rt-PA.
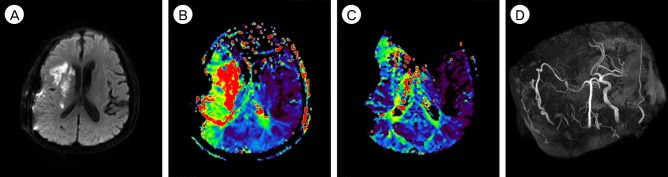
A 66-year-old man presented with cerebral infarction in the right MCA territory that manifested as left hemiparesis and dysarthria. The patient's National Institutes of Health Stroke Scale (NIHSS) score was 6 points at the time of admission. A computed tomography (CT) scan was performed immediately and revealed no acute lesion. According to the treatment protocol for acute ischemic stroke, the patient received emergent IV rt-PA (0.6 mg/kg) from a neurologist at our hospital 1 hour and 20 minutes after the onset of the event. Diffusion-weighted image (DWI) of magnetic resonance imaging (MRI) showed an acute ischemic lesion in the cortex, subcortex and corona radiata of the right hemisphere (Fig. 1A), and the DWI lesion volume was 1.7 mL. MR angiography (MRA) revealed occlusion of the right ICA (Fig. 1B). MR perfusion demonstrated decreased cerebral blood flow (CBF), slightly increased cerebral blood volume (CBV), and a large perfusion defect in the right MCA territory on the Tmax map (Fig. 1C), and the Perfusion-weighted image (Tmax > 6s) lesion volume was 128.6 mL. Because of the significant diffusion-perfusion mismatch, the patient received endovascular treatment from an interventionist 2 hours after the administration of IV rt-PA, but recanalization failed because of total cervical ICA occlusion (Fig. 1D). We initially planned to perform STA-MCA bypass the next morning because of his mild symptom and because he had received IV rt-PA. However, 8 hours after the administration of IV rt-PA, his left hemiparesis worsened from motor grade 3/4 to motor grade 1/2, and his NIHSS score was 14 at that time. The immediate follow-up MRI showed an increased cerebral infarction on DWI (Fig. 1E), and the DWI lesion volume was 6.4 mL. The operation was begun 10 hours after the administration of IV rt-PA to prevent further progression of the cerebral infarction. A successful double-barrel bypass using the frontal branch and parietal branch of the STA with a small craniotomy was achieved, as demonstrated by intraoperative microvascular Doppler sonography. Revascularization was accomplished 13.5 hours after the administration of IV rt-PA. The operation was uneventful without any difficulty associated with hemostasis, and the estimated total blood loss was less than 50 mL. An immediate postoperative CT revealed no hemorrhage and no additional cerebral infarction lesions. CT angiography revealed good patency of the bypass grafts. Oral administration of the antiplatelet and vasodilating agent cilostazol (200 mg/day) was started on postoperative day 1 to improve CBF and maintain patency of the bypass graft. After surgery, his symptom rapidly improved and his NIHSS score was 4 on postoperative day 3. MRI was performed on postoperative day 7, and revealed a slight increase in cerebral infarction (Fig. 2A) and improved perfusion status, but a perfusion defect in the right internal border zone (Fig. 2B) was still noted. The DWI lesion volume was 14.3 mL and the Tmax > 6s lesion volume was 61.9 mL. The postoperative course was uneventful and he fully recovered, his modified Rankin scale was grade 0 at the 3-months follow-up. The 1-year follow-up MRI revealed that his perfusion status had improved further (Fig. 2C) and MR angiography revealed that the STA diameters had become much thicker than the contralateral diameters (Fig. 2D). He remained well at his 1.5-year follow-up visit and continued to take cilostazol (200 mg/day).
Utilization of IV rt-PA has been the standard treatment for acute ischemic stroke. Although IV rt-PA is easily administered and is effective in some cases, the neurologic symptoms can be worsened after IV rt-PA administration if the occluded vessels do not respond to it. Bhatia et al. reported that a low rate (21.25%) of acute recanalization was observed with IV rt-PA administration in acute major vessel occlusion.2) Endovascular recanalization has been currently favored to treat major vessel occlusions unresponsive to IV rt-PA. Although endovascular treatment (EVT) has been developing and higher recanalization rates have been reported in recent trials, the rates of unsuccessful recanalization (TICI, Thrombolysis in Cerebral infarction scale ≤ 2a) were reported to be 34.3% in the REVASCAT trial16) and 41.3% in the MR CLEAN trial.12) The causes of ischemic stroke are heterogenous. An embolus responds well to thrombectomy devices, but in situ thrombosis does not respond well to them. Atherosclerotic occlusion may cause EVT failure and require additional balloon angioplasty or stenting. The natural history of acute cervical ICA occlusion with serious neurological symptom is unfavorable.1) Cervical ICA occlusion is a difficult lesion to treat in acute ischemic stroke, and Choi et al. reported that the successful recanalization rate (TICI ≥ 2b) of EVT was 36.4% in acute ischemic stroke according to cervical ICA occlusion and the rate of symptomatic intracranial hemorrhage (ICH) that resulted in death was 11.8%.3) McPherson et al. reported that early CEA can be performed safely in patients after IV rt-PA administration for acute ischemic stroke.11) However, in our case, digital subtraction angiography (DSA) showed total occlusion of the cervical ICA, which cannot be treated with CEA. Recent studies have demonstrated the benefits of using emergent STA-MCA bypass in patients with acute ischemic stroke.7)8)10)13)15) We suggest that STA-MCA bypass should be considered an alternative treatment when other reperfusion therapies are ineligible.
IV rt-PA exerts a prolonged fibrinolytic effect for more than 24 hours despite its short circulatory half-life (4-5 minutes).18) Therefore, it is recommended that invasive procedures be avoided within 24 hours after the administration of IV rt-PA. There have been rare reports of emergent STA-MCA bypass performed after IV rt-PA administration.9)17) Although a major concern was bleeding during or after surgery, it was relatively easy to achieve satisfactory hemostasis during surgery and a postoperative CT revealed no hemorrhage. Furthermore, in previous reports and in our case emergent STA-MCA bypass caused no reperfusion hemorrhage, likely owing to low-flow bypass. In our case, a remnant perfusion defect was found on follow-up MRI on postoperative day 7, and cerebral infarction had slightly increased. However, the patient showed remarkable improvement immediately after the bypass surgery. Our case suggests that the additional low-flow blood supply provided by an STA in the ischemic penumbra can minimize the progression of ischemia. The amount of flow increases over time as the bypass graft vessel matures, which is beneficial in acute ischemic stroke because it minimizes the risk of cerebral hyperperfusion syndrome.
Modern procedural descriptions of STA-MCA bypass mostly include the use of a single-branch approach. The parietal branch of the STA is usually anastomosed to either a superior division or an inferior division M4 branch, based on the gross suitability of the recipient. However, each patient develops different collateralization, which results in regional differences in perfusion. Single-barrel bypass rarely improves the perfusion status of both superior and inferior divisions of the MCA because a single branch anastomosis cannot irrigate both divisions of the MCA via retrograde filling through M1.4) Hayashi et al. described that patients had an ischemic event in the early postoperative period as a result of an insufficient increase in blood flow following the single-barrel STA-MCA bypass surgery.6) We believe that double-barrel bypass is a more suitable method for patients who have perfusion defects of both MCA divisions in acute ischemic stroke because the penumbra states are highly unstable in the acute stage of cerebral infarction.
This is the first report of emergent double-barrel STA-MCA bypass performed 10 hours after IV rt-PA administration in acute ischemic stroke. We suggest that emergent double-barrel bypass can be a viable option in patients who have perfusion defects of both MCA divisions in acute ischemic stroke after IV rt-PA administration.
References
1. Adams HP Jr, Bendixen BH, Leira E, Chang KC, Davis PH, Woolson RF, et al. Antithrombotic treatment of ischemic stroke among patients with occlusion or severe stenosis of the internal carotid artery: A report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology. 1999; 7. 53(1):122–125. PMID: 10408547.

2. Bhatia R, Hill MD, Shobha N, Menon B, Bal S, Kochar P, et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke. 2010; 10. 41(10):2254–2258. PMID: 20829513.
3. Choi JY, Lee JI, Lee TH, Sung SM, Cho HJ, Ko JK. Emergent recanalization with stenting for acute stroke due to athero-thrombotic occlusion of the cervical internal carotid artery : a single center experience. J Korean Neurosurg Soc. 2014; 6. 55(6):313–320. PMID: 25237426.

4. Duckworth EA, Rao VY, Patel AJ. Double-barrel bypass for cerebral ischemia: technique, rationale, and preliminary experience with 10 consecutive cases. Neurosurgery. 2013; 9. 73(1 Suppl Operative):ons30–ons38. discussion ones37-8. PMID: 23313980.

5. Garrett MC, Komotar RJ, Starke RM, Merkow MB, Otten ML, Sciacca RR, et al. The efficacy of direct extracranial-intracranial bypass in the treatment of symptomatic hemodynamic failure secondary to athero-occlusive disease: a systematic review. Clin Neurol Neurosurg. 2009; 5. 111(4):319–326. PMID: 19201526.

6. Hayashi T, Shirane R, Fujimura M, Tominaga T. Postoperative neurological deterioration in pediatric moyamoya disease: watershed shift and hyperperfusion. J Neurosurg Pediatr. 2010; 6(1):73–81. PMID: 20593991.

7. Horiuchi T, Nitta J, Ishizaka S, Kanaya K, Yanagawa T, Hongo K. Emergency EC-IC bypass for symptomatic atherosclerotic ischemic stroke. Neurosurg Rev. 2013; 10. 36(4):559–564. discussion 564-5. PMID: 23821132.

8. Hwang G, Oh CW, Bang JS, Jung CK, Kwon OK, Kim JE, et al. Superficial temporal artery to middle cerebral artery bypass in acute ischemic stroke and stroke in progress. Neurosurgery. 2011; 3. 68(3):723–729. discussion 729-30. PMID: 21311299.

9. Ishishita Y, Kimura T, Morita A. Urgent superficial temporal artery to middle cerebral artery bypass shortly after intravenous rt-PA. Br J Neurosurg. 2012; 10. 26(5):773–775. PMID: 22463811.

10. Lee SB, Huh PW, Kim DS, Yoo DS, Lee TG, Cho KS. Early superficial temporal artery to middle cerebral artery bypass in acute ischemic stroke. Clin Neurol Neurosurg. 2013; 8. 115(8):1238–1244. PMID: 23266265.

11. McPherson CM, Woo D, Cohen PL, Pancioli AM, Kissela BM, Carrozzella JA, et al. Early carotid endarterectomy for critical carotid artery stenosis after thrombolysis therapy in acute ischemic stroke in the middle cerebral artery. Stroke. 2001; 9. 32(9):2075–2080. PMID: 11546899.

12. Molina CA, Chamorro A, Rovira A, de Miquel A, Serena J, Roman LS, et al. REVASCAT: a randomized trial of revascularization with SOLITAIRE FR device vs. best medical therapy in the treatment of acute stroke due to anterior circulation large vessel occlusion presenting within eight-hours of symptom onset. Int J Stroke. 2015; 6. 10(4):619–626. PMID: 24206399.

13. Nussbaum ES, Janjua TM, Defillo A, Lowary JL, Nussbaum LA. Emergency extracranial-intracranial bypass surgery for acute ischemic stroke. J Neurosurg. 2010; 3. 112(3):666–673. PMID: 19499983.

14. Powers WJ, Clarke WR, Grubb RL Jr, Videen TO, Adams HP Jr, Derdeyn CP, et al. Extracranial-intracranial bypass surgery for stroke prevention in hemodynamic cerebral ischemia: the Carotid Occlusion Surgery Study randomized trial. JAMA. 2011; 306(18):1983–1992. PMID: 22068990.
15. Sakai K, Nitta J, Horiuchi T, Ogiwara T, Kobayashi S, Tanaka Y, et al. Emergency revascularization for acute main-trunk occlusion in the anterior circulation. Neurosurg Rev. 2008; 1. 31(1):69–76. discussion 76. PMID: 17957395.

16. Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015; 6. 372(24):2285–2295. PMID: 25882376.

17. Takeuchi S, Wada K, Arimoto H, Kumagai K, Osada H, Otani N, et al. Emergency superficial temporal artery to middle cerebral artery bypass after intravenous administration of tissue plasminogen activator for stroke. Turk Neurosurg. 2015; 25(4):633–637. PMID: 26242342.
18. Tanswell P, Tebbe U, Neuhaus KL, Glasle-Schwarz L, Wojcik J, Seifried E. Pharmacokinetics and fibrin specificity of alteplase during accelerated infusions in acute myocardial infarction. J Am Coll Cardiol. 1992; 4. 19(5):1071–1075. PMID: 1372625.

19. Zipfel GJ, Fox DJ Jr, Rivet DJ. Moyamoya disease in adults: the role of cerebral revascularization. Skull Base. 2005; 2. 15(1):27–41. PMID: 16148982.

Fig. 1
(A) The initial diffusion-weighted image (DWI) reveals small acute infarcts in the cortex, subcortex and corona radiata of the right hemisphere. (B) The magnetic resonance (MR) angiography shows occlusion of the right internal carotid artery (ICA) and middle cerebral artery (MCA). (C) The MR perfusion (T max map) reveals large perfusion defects in the right MCA whole territory. (D) Endovascular treatment was performed 2 hours after the administration of intravenous recombinant tissue plasminogen activator (IV rt-PA), but recanalization failed because of complete cervical ICA occlusion. (E) Eight hours after the administration of IV rt-PA, left side motor weakness of the patient worsened from motor grade 3/4 to motor grade 1/2, and the immediate follow-up DWI showed an increased cerebral infarction.
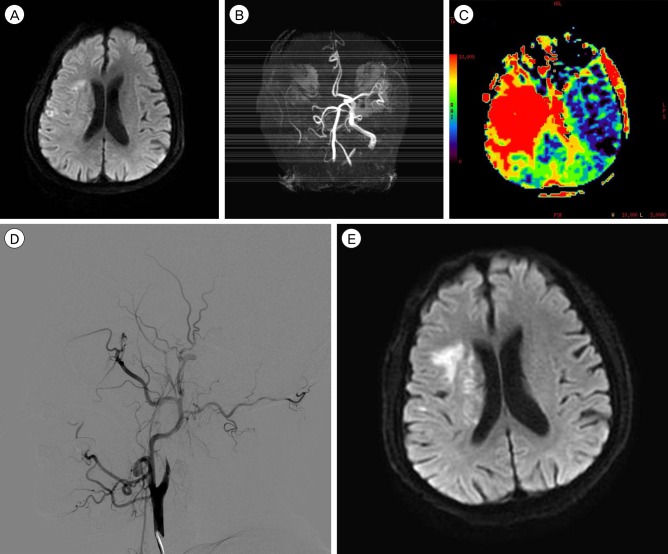
Fig. 2
(A) The diffusion-weighted image (DWI) on post-operative day 7 reveals a slight increase in cerebral infarction. (B) The postoperative 7-day magnetic resonance (MR) perfusion shows improved perfusion status, but some perfusion defects in the right internal border zone are still noted. However, his symptom rapidly improved after surgery. (C) The 1-year follow-up MR perfusion reveals more improved perfusion status. (D) The 1-year follow-up MR angiography demonstrates that the right superficial temporal artery is much thicker than its contralateral counterpart. At 3-month follow-up, the patient's mRS score was 0, and he remained well at his 1.5-year follow-up visit. mRS = modified Rankin scale.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download