Abstract
Objective
Researchers and clinicians have been unable to fully elucidate the natural course of and proper treatment for unruptured intracranial aneurysms (UIAs) smaller than or equal to 5 mm, particularly with regard to whether close observation or surgery is more appropriate. In this retrospective study, we evaluated the safety and efficacy of endovascular coil embolization of small (≤ 5 mm) asymptomatic UIAs by analyzing outcomes and complications associated with the procedure.
Materials and Methods
We analyzed data from 150 patients with small asymptomatic UIAs (≤ 5 mm) treated with coil embolization between January 2011 and December 2015. Three-dimensional angiography was used to measure aneurysm size. We evaluated procedure-related morbidity and mortality, immediate post-operative angiographic results, brain computed thomography follow-up results on post-operative day one, and clinical progress.
Results
UIAs occurred primarily in the anterior circulation area (142 cases, 94.67%), though eight patients exhibited UIAs of the posterior circulation. Following coil embolization, aneurysms with complete occlusion were observed in 137 cases (91.3%). Partial occlusion occurred in five cases (3.33%), while the procedure had failed in eight cases (5.33%). Procedure-related morbidity and mortality were five cases (3.33%) and zero cases, respectively.
Due to advancements in the field of radiology and increased public interest in neurological health, the number of patients in whom incidental unruptured intracranial aneurysms (UIA) have been identified continues to increase. However, clinicians remain divided with regard to whether close observation or surgery is most appropriate in cases of small UIA. As rupture of UIAs is associated with high morbidity and mortality, prophylactic surgery is often performed, though the risks associated with surgery should be considered.
The natural course of small UIAs remains unclear. Though the rupture rate is low, it is not zero, and there is no standard size of UIA at which treatment must begin. Juvela et al. stated that UIAs require surgical treatment regardless of their size or risk factors for rupture as long as surgery is technically possible and not contraindicated.6) In contrast, Sonobe et al. asserted that patients with single UIAs ≤ 5 mm in size should undergo careful observation due to their very low annual rupture rate. However, patients with multiple UIAs larger than 4 mm in size, patients under the age of 50, and those with a history of hypertension and/or smoking require surgical treatment due to the increased risk of rupture.5)10)12)
We analyzed data from patients treated at our hospital between January 2011 and December 2015. Over these 5 years, endovascular coil embolization was performed on a total of 324 patients. Small intracranial aneurysms (≤ 5 mm) were observed in 162 patients, 12 of whom had ruptured intracranial aneurysms. In the present retrospective study, we evaluated data from 150 patients with small asymptomatic UIAs.
Patients typically underwent computed thomography (CT) or MR angiography for evaluation of persistent symptoms such as headache or dizziness. Transfemoral cerebral angiography was then used to confirm diagnoses of suspected UIA. All embolization procedures were performed through femoral access under general anesthesia by a single skilled neurosurgeon. For complete packing, stent assisted coiling was performed in wide-neck aneurysms. Aneurysm occlusion was assessed using frontal, lateral, and working projection views. Patients underwent close observation in the intensive care unit for one day after endovascular coil embolization. Non-enhanced brain CT was performed to check for hemorrhagic or thromboembolic injury.
We categorized the results of coil embolization as complete occlusion, partial occlusion, or failed occlusion as follows. Complete occlusion was defined as occlusion of the aneurysm and aneurysm sac (Fig. 1), while partial occlusion was defined as occlusion with a space at the neck of aneurysm sac (Fig. 2).
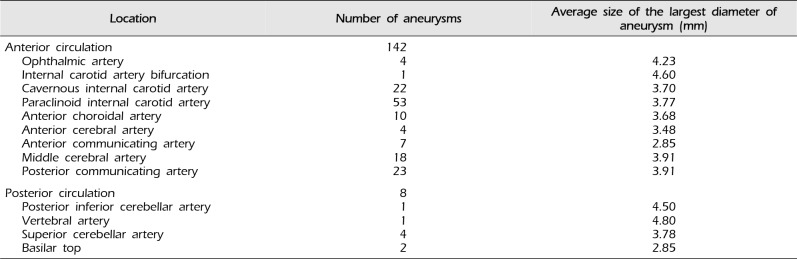
Embolization procedures were performed in 37 men (24.67%) and 113 women (75.33%). The average age of patients was 62.23 years (range: 30-83 years) (Table 1). UIAs were located primarily in the anterior circulation area (142 cases, 94.67%). The origins of aneurysms were as follows: ophthalmic artery, 4; internal carotid artery (ICA) bifurcation, 1; cavernous ICA, 22; paraclinoid ICA, 53; anterior choroidal artery, 10; anterior cerebral artery (ACA), 4; anterior communicating artery, 7; middle cerebral artery (MCA), 18; posterior communicating artery, 23; posterior inferior cerebellar artery, 1; vertebral artery, 1; superior cerebellar artery, 4; and basilar top, 2 (Table 2).
The average aneurysm sac size was 3.76 mm (range: 2.2 mm to 5 mm). UIA size was < 3 mm in 25 cases, 3-4 mm in 65 cases, and > 4 mm in 60 cases. Complete occlusion was observed in 137 cases (91.33%), while partial occlusion was observed in five cases (3.33%). The procedure had failed in eight cases (5.33%). Among these, there were three cases of coil herniation in spite of assistive stent and five cases in which microcatheter access was impaired due to severe angulation and tortuous parent vessels.
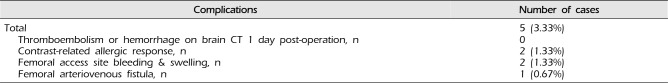
No patients experienced new-onset neurological symptoms following coil embolization. Neither hemorrhage nor cerebral infarction was observed on brain CT at 1 day post-operation. Two patients experienced bleeding and swelling at the femoral artery puncture site. In these cases, sand bags were applied at the puncture site, and patients were restricted to absolute bed rest. Ultrasonography revealed a femoral arteriovenous fistula associated with pain and mild flow sounds at the access site. Two patients experienced systemic allergic responses including rash and itching, though these symptoms resolved following steroid treatment and hydration (Table 3).
Recent advances in the sensitivity of neuroimaging techniques have increased the ability for clinicians to identify small asymptomatic UIAs. Though the rupture risk of small UIAs is quite low, rupture can be associated with devastating results such as death or brain damage from subarachnoid hemorrhage (SAH). Accordingly, preventive treatment of UIA prior to rupture has been considered necessary, though the risks of surgery must also be considered. As patients with UIAs at low risk for rupture may experience severe complications following surgery, much debate has centered around whether surgical treatment or close observation is more appropriate.
Zaccarello reported that mortality and morbidity related to surgical treatment were higher than rupture risk, with a 0.05% chance of SAH incidence for UIAs under 10 mm in patients without previous SAH history.13) In a study by Wiebers et al., 5-year cumulative rupture rates of UIAs (7 mm) in areas of the anterior circulation such as the ICA, anterior communicating artery, ACA, and MCA were 0%. However, a 2.5-fold increase in such rates was associated with UIAs in areas of the posterior circulation such as the posterior communicating artery.11)
Recently, Byoun et al.1) retrospectively evaluated the natural course of UIA progression in 1,006 patients from 2000 to 2008. Nine-year follow-up results revealed a rupture risk of 1.00%. UIAs smaller than 7 mm in patients without previous SAH history were associated with an even lower risk of rupture (0.79%). Moreover, even untreated small UIAs were often associated with a benign natural course and very low rupture risk. Nevertheless, this low risk of rupture does not definitely indicate that conservative treatment is optimal small UIAs.11) Furthermore, the retrospective nature, short follow-up period and recruitment bias of the aforementioned studies may have influenced the respective results of these studies. Indeed, may authors recommend active treatment for incidental asymptomatic UIAs due to the risk of rupture.7)
Skilled neurointerventionists can often provide successful preventive treatment for small UIAs using antiplatelet and anticoagulation therapies, which have significantly diminished morbidity rate in this patient population.8) Although a number of technically challenging procedures occurred after coil embolization in patients of the present study, procedural morbidity (five cases, 3.33%) and mortality (zero cases) were low.
Thromboembolic complications may also produce vague or mild cognitive or neurological abnormalities.2) However, several studies have revealed that endovascular coil embolization of UIAs is associated with a lower risk of such complications than surgical clipping. In a study of 130 patients with UIA, Johnston et al. reported that changes in modified Rankin Scale (mRS) scores were 25% for surgical clipping and 8% for endovascular coil embolization.3)4) Recent studies have also revealed that patients who have undergone endovascular coil embolization exhibit prognosis better than or similar to those who have undergone surgical clipping, although these studies did not consider differences in procedural morbidity and characteristics of UIA among patients.
Ogilvy and Carter reported that UIAs located in areas of the anterior circulation were associated with very low morbidity after clipping, and that long-term efficacy and durability of endovascular treatment for UIA was not yet certain.9) Therefore, further studies should investigate the ability of embolization treatment to lower rupture risk using longer clinical and neuroimaging follow-up periods and examine differences among patients according to various characteristics.
Treatment for UIAs smaller than 3 mm remains challenging, regardless of whether endovascular treatment or surgical clipping is performed. The risk of rupture for such small UIAs is high during endovascular treatment, though no such cases were observed in the present study. Some authors recommend that patients under 50 years old, those with hypertension, and those with multiple UIAs larger than 4 mm should be treated due to the high risk of rupture.5)10)12)
Much debate persists regarding the potential risk of rupture and benefits of preventive treatment for small asymptomatic UIAs. In the present study, we observed satisfactory short-term outcomes and no permanent neurologic complications following endovascular treatment of UIA. Therefore, endovascular treatment should be considered for the treatment of small asymptomatic UIAs.
References
1. Byoun HS, Huh W, Oh CW, Bang JS, Hwang G, Kwon OK. Natural history of unruptured intracranial aneurysms: a retrospective single center analysis. J Korean Neurosurg Soc. 2016; 1. 59(1):11–16. PMID: 26885281.
2. Im SH, Han MH, Kwon OK, Kwon BJ, Kim SH, Kim JE, et al. Endovascular coil embolization of 435 small asymptomatic unruptured intracranial aneurysms: procedural morbidity and patient outcome. AJNR Am J Neuroradiol. 2009; 1. 30(1):79–84. PMID: 18768715.

3. Johnston SC, Wilson CB, Halbach VV, Higashida RT, Dowd CF, McDermott MW, et al. Endovascular and surgical treatment of unruptured cerebral aneurysms: comparison of risks. Ann Neurol. 2000; 7. 48(1):11–19. PMID: 10894211.
4. Johnston SC, Zhao S, Dudley RA, Berman MF, Gress DR. Treatment of unruptured cerebral aneurysms in California. Stroke. 2001; 3. 32(3):597–605. PMID: 11239174.

5. Juvela S, Porras M, Poussa K. Natural history of unruptured intracranial aneurysms: probability and risk factors for aneurysm rupture. Neurosurg Focus. 2000; 8(5):Preview 1. PMID: 16865812.
6. Juvela S, Porras M, Poussa K. Natural history of unruptured intracranial aneurysms: probability of and risk factors for aneurysm rupture. J Neurosurg. 2000; 9. 93(3):379–387. PMID: 10969934.

7. Juvela S, Poussa K, Lehto H, Porras M. Natural history of unruptured intracranial aneurysms: a long-term follow-up study. Stroke. 2013; 9. 44(9):2414–2421. PMID: 23868274.

8. Lanterna LA, Tredici G, Dimitrov BD, Biroli F. Treatment of unruptured cerebral aneurysms by embolization with guglielmi detachable coils: case-fatality, morbidity, and effectiveness in preventing bleeding--a systematic review of the literature. Neurosurgery. 2004; 10. 55(4):767–775. discussion 775-8. PMID: 15458585.

9. Ogilvy CS, Carter BS. Stratification of outcome for surgically treated unruptured intracranial aneurysms. Neurosurgery. 2003; 1. 52(1):82–87. discussion 87-8. PMID: 12493104.

10. Sonobe M, Yamazaki T, Yonekura M, Kikuchi H. Small unruptured intracranial aneurysm verification study: SUAVe study, Japan. Stroke. 2010; 9. 41(9):1969–1977. PMID: 20671254.
11. Wiebers DO, Whisnant JP, Huston J 3rd, Meissner I, Brown RD Jr, Piepgras DG, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003; 7. 362(9378):103–110. PMID: 12867109.

12. Yonekura M. Small unruptured aneurysm verification (SUAVe Study, Japan)--interim report. Neurol Med Chir (Tokyo). 2004; 4. 44(4):213–214. PMID: 15185763.

13. Zuccarello M. Treatment strategy for patients with unruptured intracranial aneurysms. Neurol Med Chir (Tokyo). 2001; 12. 41(12):571–575. PMID: 11803581.

Fig. 1
Transfemoral cerebral angiograms in a 71-year-old male patient. The 3.6 mm × 3.3 mm unruptured intracranial aneurysm (UIA) is visible on the left posterior communicating artery. (A, B) Frontal and lateral views showing pre-embolization UIA. (C, D) Frontal and lateral views showing a totally occluded aneurysmal sac after coil embolization.
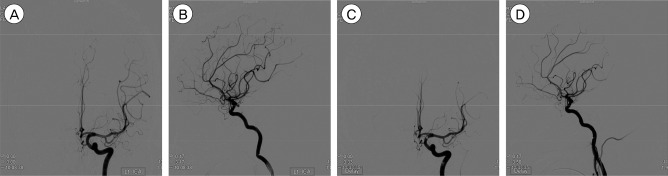
Fig. 2
Transfemoral cerebral angiograms in a 61-year-old female patient. The 4.9 mm × 2.7 mm UIA is visible on the right ophthalmic artery. (A, B) Frontal and lateral views showing pre-embolization UIA. (C, D) Frontal and lateral views showing a partially occluded aneurysmal sac after coil embolization.
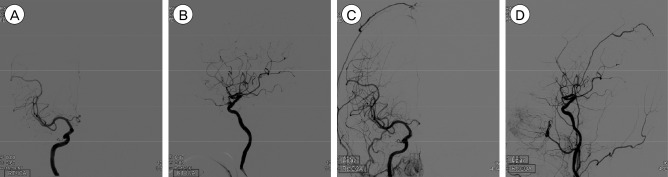
Table 1
Patient demographics
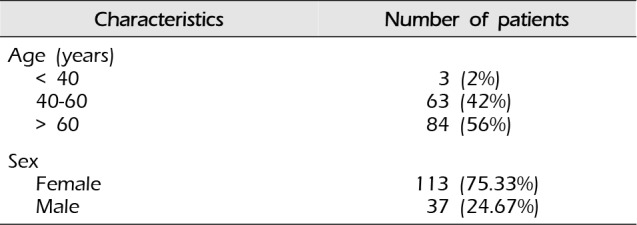
| Characteristics | Number of patients |
|---|---|
| Age (years) | |
| < 40 | 3 (2%) |
| 40-60 | 63 (42%) |
| > 60 | 84 (56%) |
| Sex | |
| Female | 113 (75.33%) |
| Male | 37 (24.67%) |
Table 2
Location and mean size of aneurysms




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download