Abstract
Objective
Spontaneous cerebellar hemorrhage (SCH) is less common than supratentorial intracerebral hemorrhage. This study investigated the treatment of SCH and the relation between its clinical and radiological manifestation and outcome.
Materials and Methods
We presented a SCH management protocol in our institute and analyzed the clinical and radiological findings in 41 SCH patients. The outcomes of each method (surgery and conservative treatment) were compared among patients with initial Glasgow Coma Scale (GCS) score of 9-13 and hematoma volume greater than 10 mL.
Results
Two (4.9%), 16 (39%), and 23 (56.1%) patients had an initial GCS score of 3-8, with 3-8, 9-13, and 14-15, respectively. Initial GCS score showed significant correlation with Glasgow Outcome Scale (GOS) score (p = 0.005). The mean largest hematoma diameter was 3.2 ± 1.5 cm, and the mean volume was 11.0 ± 11.5 mL. Both of them showed significant inverse correlation with GOS score (p < 0.001). Among patients with an initial GCS score of 9-13 and hematoma volumes greater than 10 mL, 3 (50%) had good outcome and 3 (50%) had poor outcome in the surgical, and all of those in the conservative treatment group had poor outcomes. The outcome distribution differed significantly in the surgical and conservative groups (p = 0.030).
Conclusion
Initial GCS score and largest hematoma diameter and volume on brain computed tomography are important determinants of outcome in SCH patients. The surgery group showed better outcome than the conservative treatment group among those with an intermediate neurological status and large hematomas.
Spontaneous cerebellar hemorrhages (SCHs) are rare, accounting for 6.36-16.4% of all Intracerebral hemorrhages (ICHs).1)19) Owing to the narrow space, however, even a small amount of hemorrhage can compress the brainstem, resulting in rapid deterioration of clinical symptoms, leading to dangerous consequences.22)26) For that reason, surgical treatments are more often necessary in cases of cerebellar hemorrhage compared to supratentorial ICHs, and cerebellar hemorrhages have higher mortality and morbidity rates.25) To decrease mortality and morbidity rates, a rapid diagnosis must be made and adequate treatment administered immediately. However, compared to supratentorial lesions, the risk factors related to clinical and radiological findings are not well established in cases of cerebellar hemorrhage. Since the report by Little et al.,11) the hematoma diameter has been considered a significant factor in the decision-making process for optimal treatment. We studied based on hematoma diameter as well as hematoma volume.
In this study, the relationship between clinical and radiological findings in the prognosis of patients with SCHs was compared and analyzed and we attempted to determine the factors affecting prognosis. We further investigated the management and outcome of SCHs.
The authors had 41 patients diagnosed with SCHs by brain computed tomography (CT) between January 2004 and December 2014. Cases of secondary hemorrhage resulting from a cerebral aneurysm rupture, brain tumor bleeding, arteriovenous malformations-related ICH, moyamoya disease, or hemorrhagic transformation after cerebral infarction or ICH after thrombolysis were excluded.
This study was conducted retrospectively by studying the clinical records and radiologic results of the 41 patients. The patients' conscious states were graded using the Glasgow Coma Scale (GCS), and classified into three groups: 3-8, 9-13, and 14-15, according to their GCS scores at the time of admission.
Gender, age, past medical history, consciousness at the time of admission, radiological findings, treatment methods, and prognosis were all factors that were analyzed. All patients underwent a brain CT and the highlighted components were the location, largest diameter, and volume of the hematoma, presence of intraventricular hemorrhage (IVH), presence of hydrocephalus, degree of brainstem and 4th ventricular compression, and volume expansion. The relationship between the analyzed factors and the prognosis of each case was then studied.
The largest diameter of the hematoma was defined by the longest diameter of the hematoma on brain CT. The hematoma volume was measured using equation V = A × B × C/2, where A is the longest diameter of hemorrhage on the CT section with the largest area of hemorrhage, B is the diameter perpendicular (90°) to A, and C is the number of sections with hemorrhage multiplied by the section thickness.10)
Hydrocephalus was defined as an Evans ratio greater than 0.3.14) The degree of compression on the 4th ventricle was classified according to three groups including normal, partial compression, and complete obstruction by the method used by Kirollos et al.9) (Fig. 1). Hematoma expansion within 48 hours was defined as an increase in volume of > 30% or > 6 Ml from baseline brain CT scan, using the criteria of Wada et al.24)
In our institute, the indications for surgical treatment were provided by the American Heart Association/merican Stroke Association (AHA/ASA).17) Surgery was recommended for patients who showed neurological deterioration and the maximum hematoma diameter was greater than 3cm or cerebellar hematoma volume was greater than 10 mL. When intracranial pressure was high, surgical hematoma evacuation was strongly considered. External ventricular drainage (EVD) alone was performed in patients with small hematomas who exhibited progressive mental deterioration associated with hydrocephalus caused by IVH or 4th ventricle obliteration. Patients with cerebellar hematomas less than 3 cm and without hydrocephalus, who were usually conscious and had good GCS scores, were treated conservatively. All surgeries were performed on patients in the prone position, and hematomas were removed using suboccipital craniotomy and EVD.
The treatment results were assessed using the Glasgow Outcome Scale (GOS) at discharge, and a GOS score of 4 or more was classified as a favorable result; a score of 3 or below was classified as an unfavorable result. These results were then compared with various clinical factors.
Statistical analysis was performed using SPSS version 11.0 (SPSS Inc., Chicago, IL, USA), and the Mann-Whitney U test was used for comparison of ordinal variables (GCS score, GOS score) and continuous variables (hematoma size, age) between the groups and subgroups. Pearson's chi-square test was used for comparison of categorical variables (radiographic parameters, mortality) between the groups and subgroups. p-values lower than 0.05 were considered statistically significant.
The study included 25 males and 16 females with a median age of 50.5 years (range 18-83 years). The average age of the studied patients with a cerebellar hemorrhage was 62.2 ± 14.3 years, and there were 32 patients between 50 and 80 years of age, comprising 78% of the total (Table 1).
Patients with hypertension constituted 53.7% (22 of 41), diabetes mellitus 19.5% (8 of 41), atrial fibrillation 12.2% (5 of 41), and liver disease 4.9% (2 of 41); patients on anticoagulants constituted 9.8% (4 of 41), patients on antiplatelets 14.6% (6 of 41), and those with a history of cerebro-vascular accident (CVA) 22% (9 of 41) (Table 1). Past medical history was statistically irrelevant on the GOS. Mean initial systolic blood pressure on admission was 171.0 ± 32.1 (Table 1).
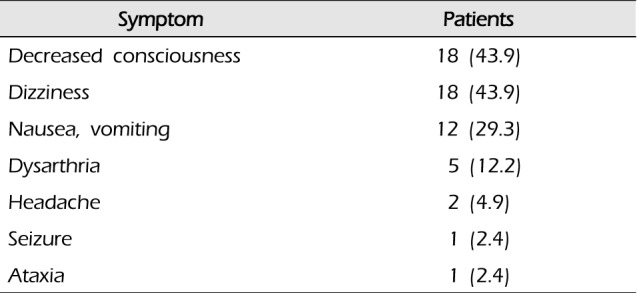
Patients with clinical symptoms of cerebellar hemorrhage included 18 with unconscious (43.9%), 18 with dizziness (43.9%), and 12 with nausea and vomiting (29.3%); dysarthria, headache, seizure, ataxia, and other symptoms were also present (Table 2). In terms of consciousness levels, 23 patients were in the good neurological status group, with scores of 14-15 (56.1%), 16 were in the intermediate group, with scores of 9-13 (39%), and 2 were in the poor group, with scores of 3-8 (4.9%) (Table 3).
The cerebellar hemorrhage was located in the cerebellar hemisphere in 30 patients (73.2%), and in the cerebellar vermis in 11 patients (26.8%). The mean largest diameter of the hematoma was 3.2 ± 1.5 cm, and 24 patients (58.5%) had hematomas larger than 3 cm. The mean volume of the hematomas was 11.0 ± 11.5 mL, and 16 patients (39%) had a hematoma volume over 10 mL (Table 1). In this study, the association between hematoma volume and largest diameter and GOS score had statistical significance (p < 0.001).
Patients with IVH on brain CT had a worse outcome than the other patients (p = 0.018). Hydrocephalus and brainstem compression detected on Brain CT was significantly more frequent in the poor outcome group (p < 0.001). All of them were independent predictors of poor outcome. Patients in whom the degree of 4th ventricular compression was higher showed poor outcome, and the association had statistical significance (p = 0.001). Hematoma expansion occurred in 2 patients in the poor outcome group, however there was no significant association with poor outcome (p = 0.073) (Table 1).
In treatment prognosis, 11 patients had a GOS score of 1 (29.3%), 4 had a score of 2 (9.8%), 1 had a score of 3 (2.4%), and 25 had a score of 5 (61%). The mortality rate was 26.8%, and the morbidity rate was 39% (Table 3).
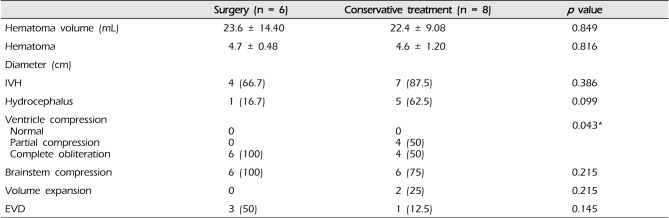
In 6 of the 14 patients with a hematoma volume greater than 10 mL and an initial GCS score of 9-13, surgical hematoma removal was performed, and extraventricular drainage was performed in 3 patients with hydrocephalus. The mean hematoma volume in patients who underwent surgical hematoma removal was 23.6 ± 14.4 mL. In the other 8 patients who underwent conservative treatment, the mean volume was 22.4 ± 9.1 mL (Table 4). The difference in degree of 4th ventricular compression between the groups who underwent surgery and who underwent conservative treatments had statistical significance (p = 0.043) (Table 4). There was no statistical significance with regard to gender, age, GCS score, hematoma volume, or hematoma diameter.
In the group with initial GCS scores 9-13 and hematoma volume was greater than 10 mL, the GOS score in the surgery group indicated good outcomes in 3 patients (50%) and poor outcome in 3 patients (50%); the GOS score in the conservative treatment group indicated poor outcomes in all 8 patients (100%). The association between surgery performance and GOS score was statistically significant (p = 0.030) (Table 5). There was no occurrence of surgery-related complications, including re-bleeding, wound infection, and pseudomeningocele.
We found that patients between the 6th and 8th decade showed the highest incidence of cerebellar hemorrhage, corresponding with the common age range for ICH incidence. Patients were predominantly male, but age and gender were not prognostic factors in our patients. This is in accordance with previous study results.8)22) The most common risk factor for cerebellar hemorrhage is hypertension, followed by coagulopathy.2)6)16) In this study, we confirm that hypertension was most common, found in 22 patients (52.4%), and 9 patients (21.4%) had a previous CVA.
Six (14.6%) patients were on antiplatelets and 4 (9.8%) were on anticoagulants. In some studies, antiplatelet and anticoagulant use was reported as a negative prognostic factor for cerebellar hemorrhage.13)14)20)23) In this study, anticoagulation was a risk factor, but not considered a prognostic factor.
The clinical symptoms for cerebellar hemorrhage are not specific and vary.4) Evidence of increased intracranial pressure (IICP), such as loss of consciousness, dizziness, nausea, and vomiting was observed in most cases, and only one case presented with cerebellar signs. No typical clinical symptoms were observed, however, owing to the specific structure of the cerebellum within the narrow posterior fossa, the clinical symptoms may deteriorate, leading to deadly consequences.
According to various studies, the location of cerebellar hemorrhage has no association with prognosis.1)21) However, in our study, hemorrhage in the cerebellar vermis was associated with a poor prognosis.
The hemorrhage in the cerebellar vermis easily compressed the 4th ventricle, leading to ventricular enlargement, and finally compressing the brainstem. This is thought to be the reason for the phenomenon.21) Regarding the prognosis of cerebellar hemorrhages, Dammann et al.4) reported that the primary conscious state of the patient is relevant. St Louis et al.22) and Wu et al.25) reported that a GCS score below 8 and the presence of hydrocephalus and IVH is related to early mortality. Cho et al.1) reported that a GCS score below 10 at admission, a hematoma volume over 15 mL, obstruction of the quadrigeminal cistern, and the presence of hydrocephalus and IVH were all negative prognostic factors for a cerebellar hemorrhage.
In this study, the factors associated with a negative prognosis of SCH were low GCS score at admission, significant hemorrhage thickness and volume, presence of hydrocephalus and IVH, presence of brainstem compression, and total occlusion of the 4th ventricle. These factors were associated with a poor prognosis (Table 1). Hematoma expansion is associated with early neurologic deterioration and is an independent predictor of poor outcome and increased morbidity.5)7) (Fig. 2). An ICH study indicated that a spot sign in the CT was strongly related to hematoma expansion.15)18) In our study, the case that showed a spot sign indicated a poor result of hematoma expansion, however there was no statistical correlation. In contrast to the larger percentage of patients who had hematoma volume expansion in the previous study, our sample population contained a small proportion of only 2 (4.9%) patients who had hematoma volume expansion.15)18) Therefore, the effect of hematoma expansion might be underestimated in our results. More studies are recommended to prove this relation.
In 1906, Ballance first reported a surgical approach to treatment of cerebellar hemorrhages.1)3) Since then, surgical treatment has become the general option for treatment of cerebellar hemorrhages.3) However, the criteria for surgery remain controversial, and many researchers have determined that a hematoma larger than 3 cm, obstruction of the quadrigeminal cistern, and compression of the 4th ventricle are surgical criteria.2)9)21) Cohen et al.2) used a maximal hematoma diameter greater than 3 cm as the surgical criterion, however, some patients with a hematoma larger than 3 cm who underwent conservative treatment had a good prognosis as well. In addition, a hematoma volume greater than 15 mL, being equivalent with a hematoma with a maximal diameter greater than 3 cm, has also been used as a criterion in some cases.1) However, we found that some patients with a hematoma volume larger than 15 mL, having undergone conservative treatment, had good prognostic outcome. Therefore, the diameter and volume of the hematoma cannot be the only surgical criteria; consciousness at admission, IVH on brain CT, presence of hydrocephalus, degree of 4th ventricular compression, and other factors should be considered indicative of surgical treatment.
In the study conducted by Cohen et al.,2) the group who underwent surgical treatment showed a worse prognosis than the conservative treatment group. However, that result was due to many factors. First, the group who underwent surgery had worse clinical and radiological findings. Second, patients in deep coma states who underwent surgery had irreversible conditions and were included in the conservative treatment group. Therefore, conduct of a randomized control study is required in order to arrive at conclusive treatments according to prognosis.
Luparello and Canavero12) reported that patients with a hematoma size larger than 3 cm and a GCS score worse than 9 showed an unfavorable outcome despite surgery, and patients with hematoma sizes smaller than 3 cm and a GCS score greater than 9 showed a favorable outcome. For patients with hematomas larger than 3 cm and a GCS score greater than 9, outcome depended on the location of the hematoma, and the concurrent presence of hydrocephalus, quadrigeminal cistern involvement, and IVH. These 14 patients with an initial GCS score of 9-13 and hematoma volume greater than 10 Ml were classified according to 2 groups based on treatment and then compared. In our study, a significant difference in GOS score was found between the group of patients who underwent surgical treatment and the group who underwent conservative treatment. We found that surgical hematoma evacuation was a dependent predictor of good outcome. Many clinical trials have failed to show an outcome benefit over conservative treatment.22) The role of surgical treatment for SCH is controversial, however, a subgroup analysis showed a potential benefit.
Patients with cerebellar hemorrhage with deteriorating neurological function or those with brainstem compression and hydrocephalus from ventricular obstruction should undergo surgical removal of the hemorrhage as soon as possible. Release of brainstem compression and relief of ventricular compression by surgical hematoma evacuation may reduce mortality and improve the treatment outcome. Limitations to this study include its retrospective nature as well as its small sample size.
In this study, the mortality rate for SCHs was 26.8%. The factors influencing the prognosis were level of consciousness at the time of admission, and the hematoma diameter and volume of the hematoma as detected on brain CT. Evidence of brainstem compression was associated with a poor prognosis.
The outcome in the surgery group was better than in the conservative treatment group, among patients with initial GCS scores of 9-13 and hematoma volume of hematoma was greater than 10 mL.
References
1. Cho SM, Hu C, Pyen JS, Whang K, Kim HJ, Han YP, et al. Predictors of outcome of spontaneous cerebellar hemorrhage. J Korean Neurosurg Soc. 1997; 10. 26(10):1395–1400.
2. Cohen ZR, Ram Z, Knoller N, Peles E, Hadani M. Management and outcome of non-traumatic cerebellar haemorrhage. Cerebrovasc Dis. 2002; 10. 14(3-4):207–213. PMID: 12403953.

3. Dahdaleh NS, Dlouhy BJ, Viljoen SV, Capuano AW, Kung DK, Torner JC, et al. Clinical and radiographic predictors of neurological outcome following posterior fossa decompression for spontaneous cerebellar hemorrhage. J Clin Neurosci. 2012; 9. 19(9):1236–1241. PMID: 22721890.

4. Dammann P, Asgari S, Bassiouni H, Gasser T, Panagiotopoulos V, Gizewski ER, et al. Spontaneous cerebellar hemorrhage--experience with 57 surgically treated patients and review of the literature. Neurosurg Rev. 2011; 1. 34(1):77–86. PMID: 20697766.

5. Davis SM, Broderick J, Hennerici M, Brun NC, Diringer MN, Mayer SA, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006; 4. 66(8):1175–1181. PMID: 16636233.

6. Dolderer S, Kallenberg K, Aschoff A, Schwab S, Schwarz S. Long-term outcome after spontaneous cerebellar haemorrhage. Eur Neurol. 2004; 9. 52(2):112–119. PMID: 15319556.

7. Han JH, Lee JM, Koh EJ, Choi HY. The spot sign predicts hematoma expansion, outcome, and mortality in patients with primary intracerebral hemorrhage. J Korean Neurosurg Soc. 2014; 10. 56(4):303–309. PMID: 25371779.

8. Hill MD, Silver FL. Epidemiologic predictors of 30-day survival in cerebellar hemorrhage. J Stroke Cerebrovasc Dis. 2001; May-Jun. 10(3):118–121. PMID: 17903811.

9. Kirollos RW, Tyagi AK, Ross SA, van Hille PT, Marks PV. Management of spontaneous cerebellar hematomas: a prospective treatment protocol. Neurosurgery. 2001; 12. 49(6):1378–1386. discussion 1386-7PMID: 11846937.

10. Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996; 8. 27(8):1304–1305. PMID: 8711791.

11. Little JR, Tubman DE, Ethier R. Cerebellar hemorrhage in adults. Diagnosis by computerized tomography. J Neurosurg. 1978; 4. 48(4):575–579. PMID: 632882.

12. Luparello V, Canavero S. Treatment of hypertensive cerebellar hemorrhage--surgical or conservative management? Neurosurgery. 1995; 9. 37(3):552–553. PMID: 7501127.

13. Matsukawa H, Shinoda M, Fujii M, Takahashi O, Yamamoto D, Murakata A, et al. Relationships among hematoma diameter, location categorized by vascular territory, and 1-year outcome in patients with cerebellar hemorrhage. World Neurosurg. 2012; Mar-Apr. 77(3-4):507–511. PMID: 22120383.

14. Matsukawa H, Shinoda M, Yamamoto D, Fujii M, Murakata A, Ishikawa R, et al. Antiplatelet agents are risk factors for cerebellar hemorrhage in patients with primary intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2011; Jul-Aug. 20(4):346–351. PMID: 20656513.

15. Moon BH, Jang DK, Han YM, Jang KS, Huh R, Park YS. Association Factors for CT Angiography Spot Sign and Hematoma Growth in Korean Patients with Acute Spontaneous Intracerebral Hemorrhage : A Single-Center Cohort Study. J Korean Neurosurg Soc. 2014; 10. 56(4):295–302. PMID: 25371778.

16. Moon KS, Park HK, Yoon SM, Bae HG, Yun IG, Choi SK. Outcomes in the management of spontaneous cerebellar hemorrhage. J Korean Neurosurg Soc. 2006; 10. 40(4):234–238.
17. Morgenstern LB, Hemphill JC 3rd, Anderson C, Becker K, Broderick JP, Connolly ES Jr, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010; 9. 41(9):2108–2129. PMID: 20651276.
18. Park SY, Kong MH, Kim JH, Kang DS, Song KY, Huh SK. Role of 'Spot Sign' on CT angiography to predict hematoma expansion in spontaneous intracerebral hemorrhage. J Korean Neurosurg Soc. 2010; 11. 48(5):399–405. PMID: 21286475.

19. Pong V, Chan KH, Chong BH, Lui WM, Leung GK, Tse HF, et al. Long-Term Outcome and Prognostic Factors After Spontaneous Cerebellar Hemorrhage. Cerebellum. 2012; 12. 11(4):939–945. PMID: 22392071.

20. Saloheimo P, Ahonen M, Juvela S, Pyhtinen J, Savolainen ER, Hillbom M. Regular aspirin-use preceding the onset of primary intracerebral hemorrhage is an independent predictor for death. Stroke. 2006; 1. 37(1):129–133. PMID: 16322483.

21. Salvati M, Cervoni L, Raco A, Delfini R. Spontaneous cerebellar hemorrhage: clinical remarks on 50 cases. Surg Neurol. 2001; 3. 55(3):156–161. discussion 161PMID: 11311913.

22. St Louis EK, Wijdicks EF, Li H, Atkinson JD. Predictors of poor outcome in patients with a spontaneous cerebellar hematoma. Can J Neurol Sci. 2000; 2. 27(1):32–36. PMID: 10676585.
23. Toyoda K, Okada Y, Minematsu K, Kamouchi M, Fujimoto S, Ibayashi S, et al. Antiplatelet therapy contributes to acute deterioration of intracerebral hemorrhage. Neurology. 2005; 10. 65(7):1000–1004. PMID: 16217049.

24. Wada R, Aviv RI, Fox AJ, Sahlas DJ, Gladstone DJ, Tomlinson G, et al. CT angiography "spot sign" predicts hematoma expansion in acute intracerebral hemorrhage. Stroke. 2007; 4. 38(4):1257–1262. PMID: 17322083.

25. Wu YT, Li TY, Chiang SL, Chu HY, Chang ST, Chen LC. Predictors of first-week mortality in patients with acute spontaneous cerebellar hemorrhage. Cerebellum. 2013; 4. 12(2):165–170. PMID: 22907124.

26. Yanaka K, Meguro K, Fujita K, Narushima K, Nose T. Immediate surgery reduces mortality in deeply comatose patients with spontaneous cerebellar hemorrhage. Neurol Med Chir (Tokyo). 2000; 6. 40(6):295–299. discussion 299-300PMID: 10892265.
Fig. 1
The degree of compression of the 4th ventricle on CT scans demonstrating different cerebellar hematomas and the appearance of the fourth ventricle on the axial CT slice with the largest transverse diameter for the hematoma. (A) Normal size and location (Grade 1). (B) Partially compressed and shifted (Grade 2). C, completely obliterated (Grade 3). CT = computed tomography.
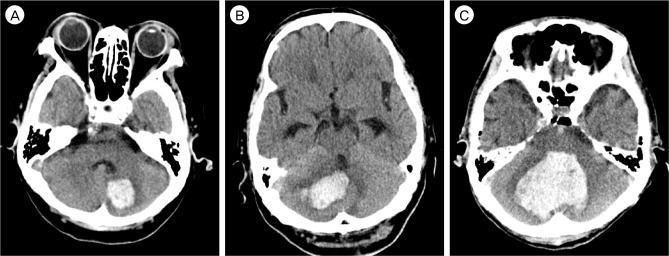
Fig. 2
Two cases of early hematoma expansion with the 'spot sign' in the source image of 3-dimensional CT angiography(3D CTA). A. Source image of 3D CTA in a 73-year-old man with mental change to stupor. B. Source image of 3D CTA in a 61-year-old man with drowsy mentality. Both images show the 'spot sign' in a cerebellar hematoma cavity. Eventually, two patients died. CT = computed tomography.
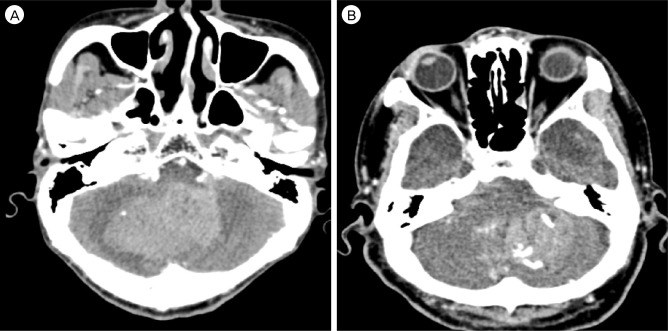
Table 1
Clinical and radiologic features of patients with spontaneous cerebellar hemorrhage: patients categorized according to outcome
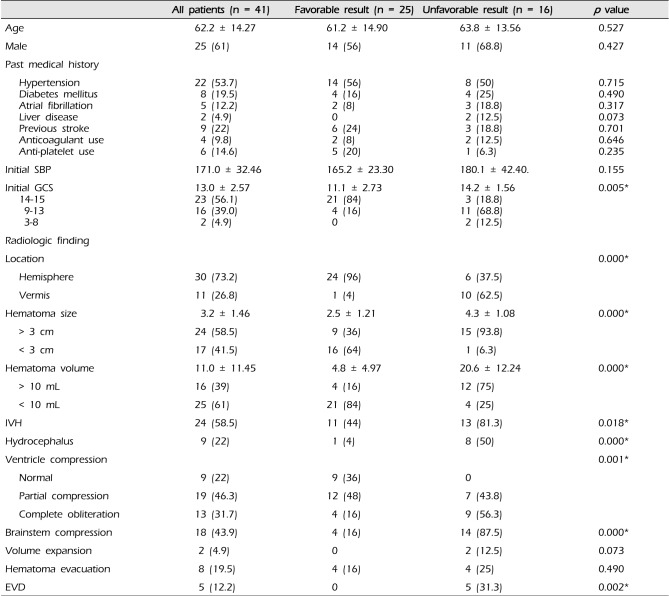
Table 2
Symptoms of patients with cerebellar hemorrhage
| Symptom | Patients |
|---|---|
| Decreased consciousness | 18 (43.9) |
| Dizziness | 18 (43.9) |
| Nausea, vomiting | 12 (29.3) |
| Dysarthria | 5 (12.2) |
| Headache | 2 (4.9) |
| Seizure | 1 (2.4) |
| Ataxia | 1 (2.4) |
Table 3
Mortality and GOS according to initial GCS
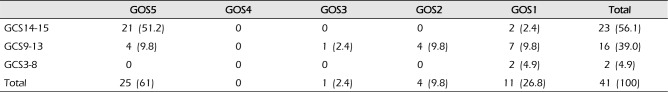
Table 4
Clinical and radiologic features of patients with cerebellar hemorrhage: patients categorized according to treatment received (9 GCS 13 and hematoma volume 10 mL)




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download