Abstract
Objective
Aneurysms originating from the proximal segment (A1) of the anterior cerebral artery are rare; however, because of their small size, the risk of injury of perforating arteries, and the location of the aneurysm in the surgical field, they are challenging to treat. We report on 15 patients with A1 aneurysms and review surgical views according to the direction of aneurysms.
Methods
Fifteen patients were diagnosed with A1 aneurysms and underwent surgical clipping or endovascular coiling at our institution between January 2006 and March 2012. We conducted a retrospective review of clinical and radiological features of all patients with A1 aneurysms.
Results
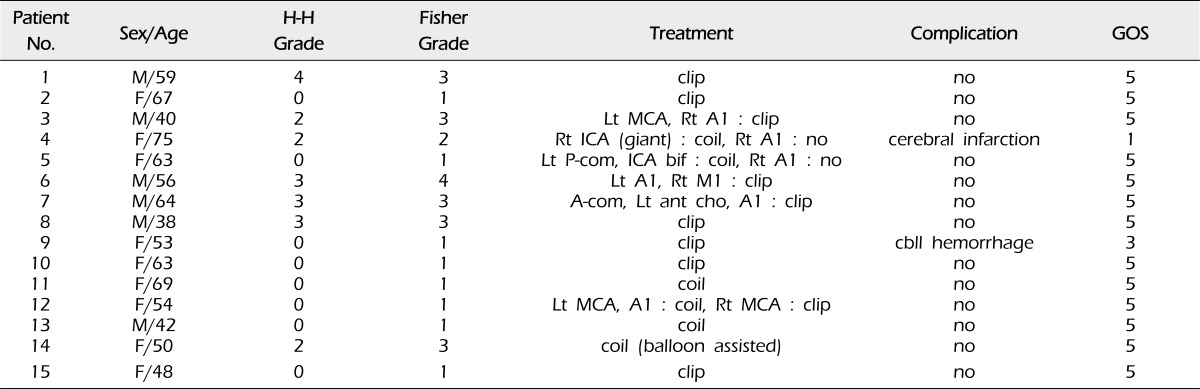
Nine patients underwent surgical clipping, and six patients received endovascular coiling. Six patients (40%) had multiple aneurysms. A1 aneurysms ranged in size from 1.5 to 8.2 mm, with an average size of 3.26 mm. Most A1 aneurysms (73%) had a posterior direction. In the surgical view, A1 aneurysms projecting posteriorly were located behind the A1 trunk. The A1 aneurysm projecting posteroinferiorly was completely eclipsed by the parent artery. In A1 aneurysms with a posterosuperior or superior direction, finding and clipping the aneurysm neck was relatively easy. Thirteen patients (87%) had an excellent outcome, one had moderate disability, and one died.
Conclusion
A1 aneurysms have certain characteristics; small size, multiple aneurysms, and, usually, a posterior direction. A1 aneurysms with a posterosuperior or superior direction are relatively easy to assess, however, clipping of A1 aneurysms with a posterior or posteroinferior direction is more difficult. Endovascular coiling is an alternative therapeutic option when surgical clipping is expected to be difficult.
The proximal segment (A1) of the anterior cerebral artery (ACA) is located between the bifurcation of the internal carotid artery (ICA) and the anterior communicating artery. A1 aneurysms comprise less than 1% of all intracranial aneurysms.3)9)14) Due to their extreme rarity, few papers on these aneurysms have been published, and, to date, only a few collected series have been reported.2)3)6)7)9)14)15)16)
The unique characteristics of A1 aneurysms include small size, fragility, multiplicity of aneurysms, and vascular anomalies, such as hypoplasia, aplasia, fenestration, duplication, and azygous ACA.2)3)6)7)9)14) Due to their small size, the risk of injury of perforating arteries, and the location of the aneurysm in the surgical field, where they are frequently located behind the parent artery, treatment of these aneurysms is a challenge.2)3)6)7)9)14) A1 aneurysms usually have a posterior direction, however, actual images of the surgical field differ according to the more specific direction of the aneurysms, such as posterosuperior or posteroinferior direction.
In this study, we report on 15 patients who were diagnosed with A1 aneurysms and treated with surgical clipping or endovascular coiling. We investigated the actual surgical views according to the direction of the aneurysms.
A total of 825 patients with aneurysms underwent surgical clipping or endovascular coiling at our institution between January 2006 and March 2012. Diagnosis of A1 aneurysms was made on the basis of three dimensional digital subtraction angiography and working projection in 15 patients, who represented 1.8% of all patients with aneurysms. There were six men and nine women, who ranged in age from 38 to 75 years (mean 56.1 years). Seven patients presented with subarachnoid hemorrhage (SAH) and eight patients were admitted with unruptured aneurysms.
Nine patients underwent surgical treatment and six patients were treated with endovascular coiling. Four A1 aneurysms of six patients treated with endovascular coiling were embolized; however, two A1 aneurysms were not treated. For the surgically treated patients, we performed standard pterional craniotomy, which opened the sylvian fissure widely and exposed the ICA, ACA and middle cerebral artery (MCA).
We conducted a retrospective review of medical records, radiological studies and operation videos of all patients with A1 aneurysms. Clinical data included initial Hunt and Hess grade, Fisher grade, modality of treatment, complication and Glasgow Outcome Scale (GOS). Radiological data included the aneurysm location, size, shape, dome direction, associated vascular anomaly, and presence of multiple aneurysms. The direction of the dome of the aneurysm was measured by the lateral angiographic planes.
We routinely made surgical views from three-dimensional digital subtraction angiography for planning of aneurysm surgery. For purposes of comparison, we investigated the actual surgical views according to the dome direction of the aneurysms. We defined cerebral infarction as a new hypodensity located in a vascular distribution on computed tomography (CT) scan.
For assessment of clinical outcomes, we used the GOS at discharge. The radiological outcomes of surgically treated patients were assessed by postoperative CT angiography 1-2 weeks after the operation. The radiological outcomes of patients treated with endovascular coiling were assessed by immediate post-embolization angiography, and magnetic resonance angiography (MRA) was used as a follow-up method for evaluation of aneurysm recanalization.
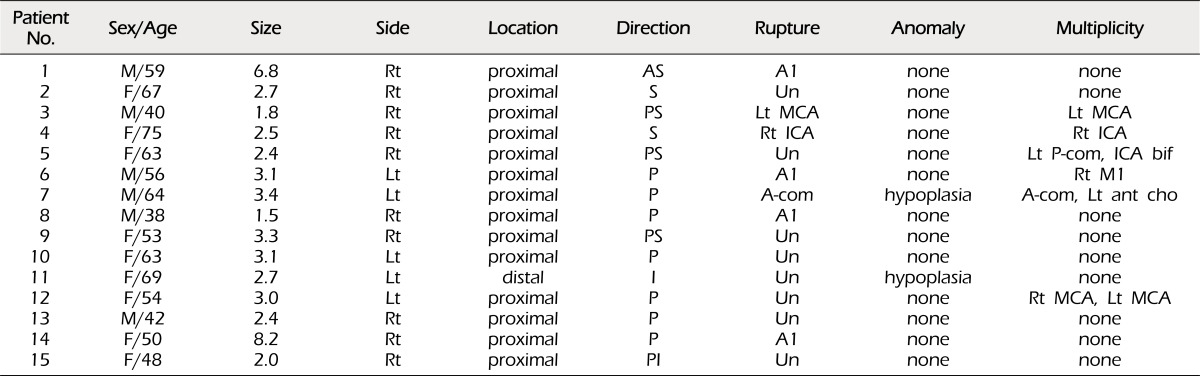
Clinical and radiological features of the patients are shown in Table 1 and Table 2. There were 15 A1 aneurysms, ten (66.7%) on the right and five (33.3%) on the left. Of these, 14 (93.3%) were found on the proximal portion and one (6.7%) was found on the distal portion. All aneurysms were saccular and ranged in size from 1.5 to 8.2 mm, with an average size of 3.26 mm. In cases of rupture, the size of the smallest aneurysm was 1.5 mm. There were no associated vascular anomalies, such as duplication, azygos ACA, or fenestration, except contralateral A1 hypoplasia in only two patients (13.3%).
Seven patients were admitted with SAH. Hunt and Hess preoperative grades were Grade 2 in three patients, Grade 3 in three patients, and Grade 4 in one patient. Six patients (40%) had multiple aneurysms. The A1 aneurysm was judged as the bleeding source in four patients and the other aneurysm was judged as the bleeding source in three patients.
Most aneurysms had a posterior or superior direction; seven aneurysms (47%) projected posteriorly, three (20%) posterosuperiorly, two (13%) superiorly, one posteroinferiorly, one anterosuperiorly and one inferiorly.
In the surgical view, the A1 aneurysm projecting posteriorly was located behind the A1 trunk. After careful dissection around the aneurysm and meticulous inspection of perforating arteries, a fenestrated clip was applied to the aneurysm parallel to the parent artery (Fig. 1). In the case of an A1 aneurysm projecting posteroinferiorly, the aneurysm was completely eclipsed by the parent artery. The aneurysm was found and clipped after mobilization of the ICA and MCA (Fig. 2). For the A1 aneurysm with a posterosuperior direction, finding the aneurysm neck and dissecting around it was relatively easy (Fig. 3).
Six patients were treated with endovascular coiling and four A1 aneurysms were embolized with detachable coils, however, two A1 aneurysms were not treated. A patient having a right ICA giant aneurysm and a right A1 aneurysm expired after endovascular coiling of the right ICA aneurysm because of cerebral infarction. In one case of a patient having multiple aneurysms (one of the left posterior communicating artery, one of the left ICA bifurcation, and one of the right A1), coiling of the A1 aneurysm failed because it disappeared during endovascular treatment. The left posterior communicating artery aneurysm and the left ICA bifurcation aneurysm were successfully embolized, however, the microcatheter kicked back out of the right A1 aneurysm during delivery of the first complex coil. The microcatheter was repositioned; however, the A1 aneurysm disappeared, perhaps due to thrombosis. Six-month follow-up MRA showed occlusion of all aneurysms and the patient has been followed up and observed in our outpatient department. Balloon-assisted coiling was used in only one case (Fig. 4).
Thirteen patients (87%) had an excellent outcome (GOS 5). One patient had severe disability because of remote cerebellar hemorrhage after surgical clipping via the pterional approach (GOS 3), and one patient died due to cerebral infarction after endovascular coiling (GOS 1). Postoperative CT revealed a small area of low density in the genu portion of the internal capsule in two patients who underwent surgical clipping, perhaps due to perforator injuries. Fortunately, they had no neurological deficits or symptoms (Fig. 5).
Aneurysms arising from A1 segments are usually small and appear to rupture at a smaller size than other aneurysms.3)9) The average size of ruptured A1 aneurysms in our series was 3.26 mm and the size of the smallest aneurysm in our ruptured cases was 1.5 mm. In several series, the sizes of most A1 aneurysms in ruptured cases have been less than 7 mm.2)3)7)9) A1 aneurysms are usually small, with a fragile wall. Unruptured A1 aneurysms require surgical clipping or endovascular coiling, even when they are small.
A1 aneurysms have some characteristics that are usually associated with vascular anomalies and they have a high incidence of multiplicity (25-70%).2)3)7)9)14) In our series, the incidence of multiple aneurysms was high (40%), however, there were no vascular anomalies, except for contralateral A1 hypoplasia in only two patients (15.3%).
The radiologic appearance of A1 aneurysms resembles that of internal carotid bifurcation or anterior communicating artery aneurysms. In surgical planning, analysis of patients' angiography and correct assessment of the origin of the aneurysm neck is important. Most perforating arteries are very thin, and they cannot be adequately visualized on digital subtraction angiography or CT angiography. The number of perforating arteries ranges from two to 15, with an average of eight.11) Most perforating arteries arise from the proximal half of the A1 trunk, usually originating from the superior or posterior aspect of the A1 trunk.11)13) They supply the anterior commissure, anterior hypothalamus, genu of the internal capsule, and anterior parts of the caudate nucleus and the globus pallidus.4)11)13)
Most A1 aneurysms (73%) in our series had a posterior direction, as reported in other studies.2)3)6)7)9)14) In cases of aneurysms projecting posteriorly, access and preservation of perforating arteries is difficult, because they are usually located behind the parent artery in the operative field. Clipping of the A1 aneurysm projecting posteroinferiorly was more difficult, because it was completely eclipsed by the parent artery. However, in A1 aneurysms with a posterosuperior or superior direction, finding the aneurysm neck and dissecting around it was relatively easy.
Damage or occlusion of perforating arteries could result from direct injury or clip deviation after removal of brain retractors. To prevent occlusion of perforating arteries, some considerations should be given. Additional supraorbital craniotomy facilitates exposure without significant retraction of the frontal lobe. And, it is important to open the Sylvian fissure widely enough to mobilize the MCA and ICA, which improves the visualization of the structure behind the A1 trunk. In addition, the smallest possible clip should be selected and the deviation of the clip and kinking of the artery should be checked after removal of brain retractors.
Various methods of intraoperative monitoring, such as Doppler ultrasonography, intraoperative angiography, intraoperative indocyanine green (ICG) fluorescence, and electrophysiologic monitoring of evoked potentials can be used during the operation to confirm the patency of the parent artery or perforating arteries.12) In general, we used 20-MHz probes in microvascular Doppler flowmetry, which can detect blood flow noninvasively. However, placement of the probe on target vessels in the deep surgical field and detection of small perforating arteries is difficult. ICG fluorescent imaging is helpful in evaluation of vessel patency and aneurysm occlusion after clip placement. Functional monitoring using somatosensory- and motor-evoked potentials and intraoperative angiography is also helpful for preservation of perforators if they could be used during the operation.
Endovascular coiling of A1 aneurysms is challenging because they are usually small and mostly project posteriorly and they are primarily located in the just proximal portion of the A1 segment. In the past, surgical clipping has mainly been performed for treatment of patients with A1 aneurysms. However, endovascular coiling has been used increasingly in recent years and recent articles on endovascular treatment of A1 aneurysms have reported good results.1)5)8)10) A1 aneurysms are less prone to recanalization due to their small size and sidewall aneurysms.10) Endovascular coiling is an alternative therapeutic option for management of A1 aneurysms.
This study has several limitations. The total number of cases is small and coil embolization was not performed in two A1 aneurysms of all patients treated with endovascular treatment. To date, due to the rarity of A1 aneurysms, only a few collected cases have been reported. Conduct of multicenter studies is required in order to understand characteristics of A1 aneurysms and for development of a proper treatment plan.
A1 aneurysms have unique characteristics, including small size, multiplicity, and, usually, a posterior dome direction; and A1 aneurysms are found primarily on the proximal portion of the A1 segment and they usually have a posterior or superior direction. For A1 aneurysms with a posterosuperior or superior direction, finding and clipping the aneurysm neck is relatively easy. However, in the case of aneurysms having a posterior direction, it is difficult to achieve good access because they are located behind the parent artery. In addition, clipping of the A1 aneurysm projecting posteroinferiorly is more difficult, because it is completely eclipsed by the parent artery. Endovascular coiling should be proposed as an alternative therapeutic option when the endovascular approach is available and surgical clipping is expected to be difficult.
References
1. Chang HW, Youn SW, Jung C, Kang HS, Sohn CH, Kwon BJ, et al. Technical strategy in endovascular treatment of proximal anterior cerebral artery aneurysms. Acta Neurochir (Wien). 2011; 2. 153(2):279–285. PMID: 20872259.

2. Czepko R, Libionka W, Lopatka P. Characteristics and surgery of aneurysms of the proximal (A1) segment of the anterior cerebral artery. J Neurosurg Sci. 2005; 9. 49(3):85–95. PMID: 16288191.
3. Dashti R, Hernesniemi J, Lehto H, Niemelä M, Lehecka M, Rinne J, et al. Microneurosurgical management of proximal anterior cerebral artery aneurysms. Surg Neurol. 2007; 10. 68(4):366–377. PMID: 17905060.

4. Dunker RO, Harris AB. Surgical anatomy of the proximal anterior cerebral artery. J Neurosurg. 1976; 3. 44(3):359–367. PMID: 1249614.

5. Gupta R, Horowitz MB, Gilman S. Neuroform stent-assisted coil embolization of a ruptured A1 segment anterior cerebral artery aneurysm. J Neuroimaging. 2006; 4. 16(2):117–119. PMID: 16629732.

6. Handa J, Nakasu Y, Matsuda M, Kyoshima K. Aneurysms of the proximal anterior cerebral artery. Surg Neurol. 1984; 11. 22(5):486–490. PMID: 6495158.

7. Hino A, Fujimoto M, Iwamoto Y, Oka H, Echigo T. Surgery of proximal anterior cerebral artery aneurysms. Acta Neurochir (Wien). 2002; 12. 144(12):1291–1296. discussion 1296. PMID: 12478340.
8. Lee HY, Ahn JS, Suh DC, Lee DH. Z-shaped microcatheter tip shaping for embolization of aneurysms at the proximal A1 segment of the anterior cerebral artery: a technical note. Neurointervention. 2011; 8. 6(2):95–99. PMID: 22125756.

9. Lee JM, Joo SP, Kim TS, Go EJ, Choi HY, Seo BR. Surgical management of anterior cerebral artery aneurysms of the proximal (A1) segment. World Neurosurg. 2010; Oct-Nov. 74(4-5):478–482. PMID: 21492598.

10. Lubicz B, Bruneau M, Dewindt A, Lwfranc F, Baleriaux D, De Whitte O. Endovascular treatment of proximal anterior cerebral artery aneurysms. Neuroradiology. 2009; 2. 51(2):99–102. PMID: 18985332.

11. Perlmutter D, Rhoton AL Jr. Microsurgical anatomy of the anterior cerebral-anterior communicating-recurrent artery complex. J Neurosurg. 1976; 9. 45(3):259–272. PMID: 948013.

12. Raabe A, Beck J, Gerlach R, Zimmermann M, Seifert V. Near-infrared indocyanine green video angiography: a new method for intraoperative assessment of vascular flow. Neurosurgery. 2003; 1. 52(1):132–139. discussion 139. PMID: 12493110.

13. Rosner SS, Rhoton AL Jr, Ono M, Barry M. Microsurgical anatomy of the anterior perforating arteries. J Neurosurg. 1984; 9. 61(3):468–485. PMID: 6747683.

14. Suzuki M, Onuma T, Sakurai Y, Mizoi K, Ogawa A, Yoshimoto T. Aneurysms arising from the proximal (A1) segment of the anterior cerebral artery. A study of 38 cases. J Neurosurg. 1992; 3. 76(3):455–458. PMID: 1738027.
15. Wakabayashi T, Tamaki N, Yamashita H, Saya H, Suyama T, Matsumoto S. Angiographic classification of aneurysms of the horizontal segment of the anterior cerebral artery. Surg Neurol. 1985; 7. 24(1):31–34. PMID: 4012555.

16. Wanibuchi M, Kurokawa Y, Ishiguro M, Fujishige M, Inaba K. Characteristics of aneurysms arising from the horizontal portion of the anterior cerebral artery. Surg Neurol. 2001; 3. 55(3):148–154. discussion 154-5. PMID: 11311909.

Fig. 1
A: Left carotid angiogram shows a left A1 aneurysm with a posterior projection. B: The surgical view from three-dimensional digital subtraction angiography was made for planning of aneurysm surgery C: Intraoperative photograph shows an A1 aneurysm (thick arrow) located behind the A1 trunk and a perforating artery (thin arrow). D: A fenestrated clip was applied to the aneurysm parallel to the parent artery.
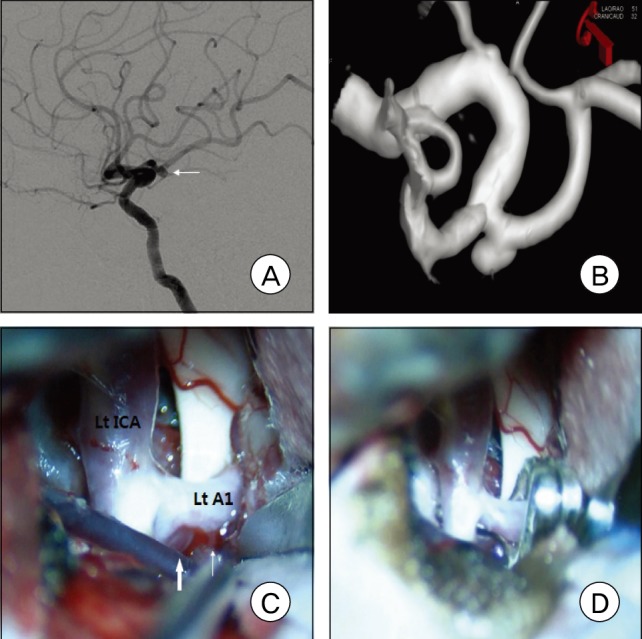
Fig. 2
A: Right carotid angiogram shows a right A1 aneurysm with a posteroinferior projection. B: Intraoperative photograph shows that the aneurysm was completely eclipsed by the parent artery, as shown in the surgical view from three-dimensional digital subtraction angiography. C and D: The aneurysm was found and clipped after mobilization of the internal carotid artery and middle cerebral artery.
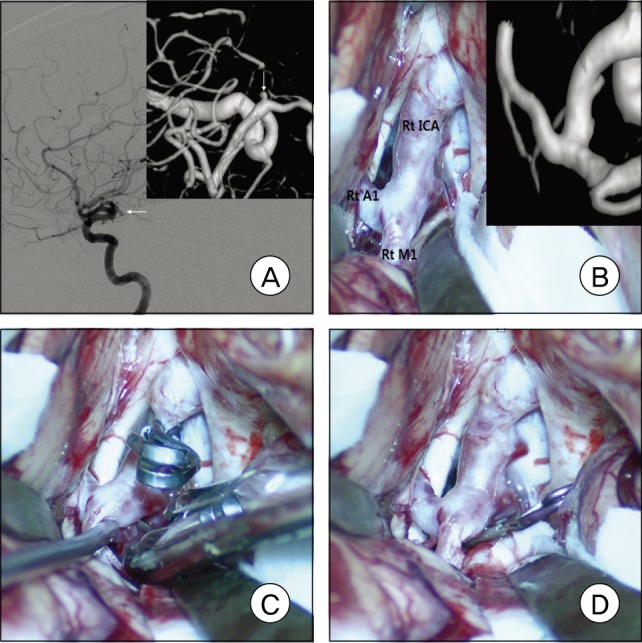
Fig. 3
A and B: Right carotid angiograms show a right A1 aneurysm with a posterosuperior projection. C and D: Intraoperative photographs show that finding and clipping the aneurysm neck was relatively easy.
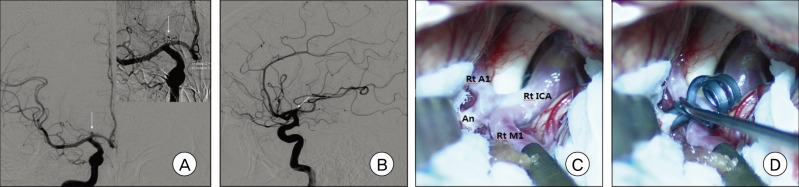
Fig. 4
A and B: Right carotid angiograms show a right A1 aneurysm with a posterior projection. C: The microcatheter kicked back out of the right A1 aneurysm during delivery of the first complex coil. D: Balloon-assisted coil embolization technique was used.
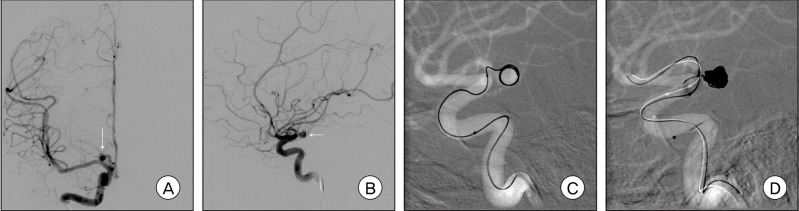
Fig. 5
A: Left carotid angiogram shows multiple A1 aneurysms (one of the anterior communicating artery, one of the left anterior choroidal artery, and one of the left A1). B: Left carotid angiogram shows a left A1 aneurysm. C and D: Postoperative computed tomography scans show a small area of low density in the genu portion of the internal capsule in each patient (A and B).
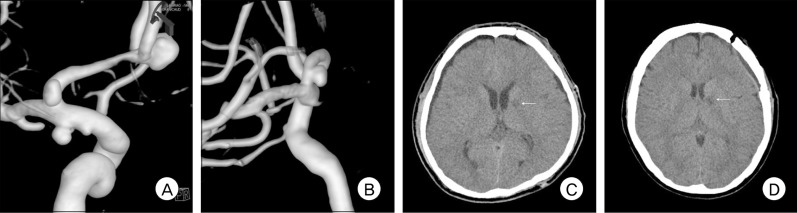
Table 1
Radiological features of the patients
M= male; F= female; Rt= right; Lt= left; AS= anterosuperior, S= superior; P= posterior; PS= posterosuperior; I= inferior; PI= posteroinferior; Un= unruptured; MCA= middle cerebral artery; ICA= internal cerebral artery; A-com= anterior communicating artery; P-com= posterior communicating artery; bif= bifurcation; ant cho= anterior choroidal artery




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download