Abstract
Acute upper gastrointestinal bleeding is a common medical emergency around the world and the major cause is peptic ulcer bleeding. Endoscopic treatment is fundamental for the management of peptic ulcer bleeding. Despite recent advances in endoscopic treatment, mortality from peptic ulcer bleeding has still remained high. This is because the disease often occurs in elderly patients with frequent comorbidities and are taking ulcerogenic medications. Therefore, the management of peptic ulcer bleeding is still a challenge for clinicians. This article reviews the various endoscopic methods available for management of peptic ulcer bleeding and the techniques in using these methods.
Acute upper gastrointestinal bleeding (UGIB) is defined as bleeding arising from a lesion proximal to the ligament of Treitz.1 Peptic ulcer bleeding (PUB) is reported to be the most common cause of UGIB.2 The incidence of PUB is reported to be from 19.4 to 57 per 100,000 population per year.3 The mortality rate from PUB is reported to be from 2.5% to 5.8%.4,5,6 In United Kingdom, surgery for PUB has continued to decrease from 8% to 2% between 1993 and 2006.2,7 In the USA, admissions to hospitals for PUB decreased by 28.2% and the use of endoscopic treatment increased by 58.9% between 1993 and 2006.8 In Korea, an early study reported that the average age of patients with PUB increased from 50.8 to 56.5 years between 1993 to 1995 period and 2000 to 2002 period.9 The rate of endoscopic treatment increased from 17.6% to 39.0% and the rate of surgery decreased from 11.5% to 5.2% during this period. However, the overall mortality rate increased from 5.4% to 7.7% during this period. This is because the disease often occurs in elderly patients with frequent comorbidities and are taking ulcerogenic medications. Currently, most of the studies on the epidemiologic data on PUB have been published from Western countries. Although peptic ulcer disease is common in Korea, the prevalence of PUB has not been well studied. This review focuses on the endoscopic management of PUB. Pre-endoscopic assessment, resuscitation and postendoscopic management are beyond the scope of this review and is not touched upon in this review.
Complete visualization of the stomach may not be possible in patients with PUB due to stomach contents and blood. A clear endoscopic field is essential for the success of endoscopic hemostasis. Prokinetic drugs such as erythromycin and metoclopramide given before endoscopy were expected to improve visualization. However, the results of currently published studies are disappointing. A recent meta-analysis found that prokinetic drugs reduced the need for a second endoscopic examination; however, there was no difference in need for transfusion, hospital stay, and surgery.10 However, only three full text articles were included in this meta-analysis and currently there is not enough evidence regarding the use of prokinetics in patients suspected with PUB.
Endoscopy is essential for the diagnosis of the cause of bleeding and endoscopic treatment can reduce re-bleeding, surgery, and mortality.11 Although the role of endoscopy is obvious in PUB patients, when to perform endoscopy is not yet clarified. The majority of data published suggest that early endoscopy (generally defined as endoscopy within 24 hours) is safe and can reduce transfusion requirements and length of hospital admission.12 However, no mortality benefit could be identified with early endoscopy. Several studies have investigated the efficacy of urgent endoscopy (endoscopy performed with 6 or 12 hours) compared with elective endoscopy.13,14,15 The results of these studies suggest that urgent endoscopy can reduce hospital stay and amount of blood transfusion. However, outcomes of recurrent bleeding, surgery, and deaths were not associated with urgent endoscopy. Based on these results, early endoscopy within 24 hours seems to be mandatory for patients with PUB while urgent endoscopy within 1 to 4 hours is recommended for patients with clinical evidence of continued bleeding.
The Forrest classification is most widely used to classify the endoscopic appearance of bleeding peptic ulcers.16 Nowadays it is widely used to predict the risk of re-bleeding and mortality and is known to have a stronger association with gastric ulcers compared with duodenal ulcers.17,18,19 The Forrest classification classifies ulcers with a spurting hemorrhage (Forrest Ia), an oozing hemorrhage (Forrest Ib), a visible vessel (Forrest IIa), an adherent clot (Forrest IIb), hematin on the ulcer base (Forrest IIc), and a clean ulcer base (Forrest III).19
Endoscopic treatment is mandatory for ulcers with active bleeding or with a non-bleeding visible vessel.20 Endoscopic treatment is generally not recommended for ulcers with hematin on the ulcer base or a clean ulcer base.20 Controversy exists whether ulcers with an adherent clot need endoscopic treatment, but treatment is generally recommended when the clot is resistant to vigorous irrigation.20
Endoscopic treatment can be divided into injection, thermal, and mechanical methods. Injection therapies consists of epinephrine (Fig. 1), sclerosants (absolute ethanol, polidocanol), and tissue adhesives (thrombin/fibrin glues). Injection of epinephrine is the most widely used method for hemostasis. It is easy to perform and requires less coordination between the endoscopist and the assistant compared to other methods. Epinephrine injection is effective at achieving initial hemostasis mainly due to a tampon effect.1 However, epinephrine monotherapy is less effective than other monotherapies such as electrocoagulation, clips, or fibrin glues in preventing further bleeding.21 Also, adding a second modality such as electrocoagulation or clips is significantly more effective than epinephrine alone in reducing further bleeding and surgery.21 It is now widely considered that epinephrine monotherapy is inadequate and should be combined with another modality. In clinical practice, injection of epinephrine is generally performed before other therapies in order to slow or stop bleeding which allows improved visualization for subsequent therapies.20 The optimal volume of epinephrine injection is not known; however, diluted solutions (1:10,000 or 1:20,000) are injected in 0.2 to 2 mL aliquots in all four quadrants of the bleeding stigma. Sclerosant injection significantly reduces further bleeding when compared with no endoscopic therapy.21 However, sclerosants such as alcohol is not routinely used for treatment of bleeding peptic ulcers, possibly due to concerns for tissue necrosis. Absolute alcohol can be administered in 0.1 to 0.2 mL aliquots with limitations of 1 to 2 mL due to concerns of tissue injury.22
Thermal contact therapy can be classified into contact or noncontact methods. Contact methods consists of heater probe or bipolar electrocoagulation and noncontact methods consist of argon plasma coagulation (Fig. 2).23 Thermal methods are significantly effective in achieving initial hemostasis, reducing further bleeding, surgery, and mortality.21 Currently, no significant difference has been found between the different thermal modalities. Two studies reported that epinephrine injection followed by thermal therapy was more effective than thermal therapy alone.24,25 Thermal contact therapy should be performed with the tip of the probe as close as possible to the bleeding ulcer. Endoscopic caps may be used to improve visualization and access for therapy.26 When available, use of soft caps is preferred to minimize contact bleeding.
Clips have been found to be more effective than epinephrine injection in reducing further bleeding and surgery but less effective than thermal therapy (Fig. 3).21 Clips likely do not induce tissue injury which would be a benefit over thermal therapies and sclerosants. However, clips are not currently reimbursed by the Korean National Health Insurance and is generally considered to be more expensive than the other hemostatic methods. Also, training the assistants in handling of the clip is needed for successful application. Another limitation of endoclips is that it is difficult to apply in fibrotic lesions.27 Also, currently used endoclips allow for only single clip deployment and in cases where multiple clips are necessary, repeated passage of the device may prolong the procedure time. When using an endoclip, the clip and the target should be close to the endoscope for better control of the clip. In order to capture maximal amount of tissue around the lesion, suction can be applied before deployment.27 Precise placement of the first endoclip is crucial as improper placement can prevent the proper placement of additional clips.
Second look endoscopy is defined as routine endoscopy after initial hemostatic therapy. According to recent guidelines, routine second look endoscopy after initial endoscopic hemostasis is not recommended and should be reserved for patients with high risk of re-bleeding.20 This is because high dose intravenous proton pump inhibitor was shown to be as effective as second look endoscopy in reducing re-bleeding.28 However, there is no grading system that can reliably classify patients who are at high risk of re-bleeding and further studies are needed in this aspect.29
Endoscopic hemostasis is not successful in 8% to 15% of patients.1 The re-bleeding rate of patients with ulcers is dependent on factors such as size, depth, location, concurrent medical comorbidities, severe coagulopathies, presentation with shock or hypotension, and start of bleeding as an inpatient.30 Most of the re-bleeding episodes are reported to occur in the first 7 days.31,32 Patients who fail to achieve hemostasis by endoscopic measure should receive angiographic embolisation. When compared to surgery, angiographic embolisation is associated with reduced complications but with higher re-bleeding rates.33,34,35,36,37
Recurrent bleeding occurring after initial successful endoscopic hemostasis can be treated by repeated endoscopic treatment. When compared to surgery, repeated endoscopic treatment achieves comparable hemostasis with substantially less postoperative complications.38 Risk factors reported to be associated with re-bleeding are nonsteroidal anti-inflammatory drug use, ulcer size, age, genetic components, anticoagulant use, shock, and low blood pressure.3 Currently, a nationwide cohort investigating the risk factors associated with re-bleeding after PUB in Korea is ongoing.
The epidemiology of PUB has changed and patients with PUB presents at an older age with increased comorbidities. Despite advances in pharmacology and endoscopic techniques, the mortality rate of PUB seems to be similar partly due to this change in epidemiology. Endoscopy remains the mainstay of treatment for patients with PUB. Among the various hemostatic methods, epinephrine injection should be combined with another modality. The benefits of routine second look endoscopy seem to be minimal and patients who fail to achieve initial hemostasis should receive angiographic embolisation.
References
1. Lau JY, Barkun A, Fan DM, Kuipers EJ, Yang YS, Chan FK. Challenges in the management of acute peptic ulcer bleeding. Lancet. 2013; 381:2033–2043. PMID: 23746903.

2. Hearnshaw SA, Logan RF, Lowe D, Travis SP, Murphy MF, Palmer KR. Acute upper gastrointestinal bleeding in the UK: patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut. 2011; 60:1327–1335. PMID: 21490373.

3. Lau JY, Sung J, Hill C, Henderson C, Howden CW, Metz DC. Systematic review of the epidemiology of complicated peptic ulcer disease: incidence, recurrence, risk factors and mortality. Digestion. 2011; 84:102–113. PMID: 21494041.

4. Barkun A, Sabbah S, Enns R, et al. The Canadian Registry on Nonvariceal Upper Gastrointestinal Bleeding and Endoscopy (RUGBE): endoscopic hemostasis and proton pump inhibition are associated with improved outcomes in a real-life setting. Am J Gastroenterol. 2004; 99:1238–1246. PMID: 15233660.

5. Laine L, Yang H, Chang SC, Datto C. Trends for incidence of hospitalization and death due to GI complications in the United States from 2001 to 2009. Am J Gastroenterol. 2012; 107:1190–1195. PMID: 22688850.

6. Marmo R, Koch M, Cipolletta L, et al. Predictive factors of mortality from nonvariceal upper gastrointestinal hemorrhage: a multicenter study. Am J Gastroenterol. 2008; 103:1639–1647. PMID: 18564127.
7. Rockall TA, Logan RF, Devlin HB, Northfield TC. Incidence of and mortality from acute upper gastrointestinal haemorrhage in the United Kingdom. Steering Committee and members of the National Audit of Acute Upper Gastrointestinal Haemorrhage. BMJ. 1995; 311:222–226. PMID: 7627034.
8. Wang YR, Richter JE, Dempsey DT. Trends and outcomes of hospitalizations for peptic ulcer disease in the United States, 1993 to 2006. Ann Surg. 2010; 251:51–58. PMID: 20009753.

9. Choi JW, Kim HY, Kim KH, et al. Has any improvement been made in the clinical outcome of patients with bleeding peptic ulcer in the part 10 years? Korean J Gastrointest Endosc. 2005; 30:235–242.
10. Barkun AN, Bardou M, Martel M, Gralnek IM, Sung JJ. Prokinetics in acute upper GI bleeding: a meta-analysis. Gastrointest Endosc. 2010; 72:1138–1145. PMID: 20970794.

11. Cook DJ, Guyatt GH, Salena BJ, Laine LA. Endoscopic therapy for acute nonvariceal upper gastrointestinal hemorrhage: a meta-analysis. Gastroenterology. 1992; 102:139–148. PMID: 1530782.

12. Spiegel BM, Vakil NB, Ofman JJ. Endoscopy for acute nonvariceal upper gastrointestinal tract hemorrhage: is sooner better? A systematic review. Arch Intern Med. 2001; 161:1393–1404. PMID: 11386888.
13. Lin HJ, Wang K, Perng CL, et al. Early or delayed endoscopy for patients with peptic ulcer bleeding. A prospective randomized study. J Clin Gastroenterol. 1996; 22:267–271. PMID: 8771420.
14. Lee JG, Turnipseed S, Romano PS, et al. Endoscopy-based triage significantly reduces hospitalization rates and costs of treating upper GI bleeding: a randomized controlled trial. Gastrointest Endosc. 1999; 50:755–761. PMID: 10570332.

15. Bjorkman DJ, Zaman A, Fennerty MB, Lieberman D, Disario JA, Guest-Warnick G. Urgent vs. elective endoscopy for acute non-variceal upper-GI bleeding: an effectiveness study. Gastrointest Endosc. 2004; 60:1–8. PMID: 15229417.

16. Forrest JA, Finlayson ND, Shearman DJ. Endoscopy in gastrointestinal bleeding. Lancet. 1974; 2:394–397. PMID: 4136718.

17. Guglielmi A, Ruzzenente A, Sandri M, et al. Risk assessment and prediction of rebleeding in bleeding gastroduodenal ulcer. Endoscopy. 2002; 34:778–786. PMID: 12244498.

18. Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. 1996; 38:316–321. PMID: 8675081.

19. de Groot NL, van Oijen MG, Kessels K, et al. Reassessment of the predictive value of the Forrest classification for peptic ulcer rebleeding and mortality: can classification be simplified? Endoscopy. 2014; 46:46–52. PMID: 24218308.

20. Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012; 107:345–360. PMID: 22310222.

21. Laine L, McQuaid KR. Endoscopic therapy for bleeding ulcers: an evidence-based approach based on meta-analyses of randomized controlled trials. Clin Gastroenterol Hepatol. 2009; 7:33–47. PMID: 18986845.

22. Laine L. Multipolar electrocoagulation versus injection therapy in the treatment of bleeding peptic ulcers. A prospective, randomized trial. Gastroenterology. 1990; 99:1303–1306. PMID: 2210238.
23. Kim KB, Yoon SM, Youn SJ. Endoscopy for nonvariceal upper gastrointestinal bleeding. Clin Endosc. 2014; 47:315–319. PMID: 25133117.

24. Bianco MA, Rotondano G, Marmo R, Piscopo R, Orsini L, Cipolletta L. Combined epinephrine and bipolar probe coagulation vs. bipolar probe coagulation alone for bleeding peptic ulcer: a randomized, controlled trial. Gastrointest Endosc. 2004; 60:910–915. PMID: 15605005.

25. Lin HJ, Tseng GY, Perng CL, Lee FY, Chang FY, Lee SD. Comparison of adrenaline injection and bipolar electrocoagulation for the arrest of peptic ulcer bleeding. Gut. 1999; 44:715–719. PMID: 10205211.

28. Tsoi KK, Chan HC, Chiu PW, Pau CY, Lau JY, Sung JJ. Second-look endoscopy with thermal coagulation or injections for peptic ulcer bleeding: a meta-analysis. J Gastroenterol Hepatol. 2010; 25:8–13. PMID: 20136971.

29. Elmunzer BJ, Young SD, Inadomi JM, Schoenfeld P, Laine L. Systematic review of the predictors of recurrent hemorrhage after endoscopic hemostatic therapy for bleeding peptic ulcers. Am J Gastroenterol. 2008; 103:2625–2632. PMID: 18684171.

30. Jensen DM, Machicado GA. Endoscopic hemostasis of ulcer hemorrhage with injection, thermal, and combination methods. Tech Gastrointest Endosc. 2005; 7:124–131.

31. Bini EJ, Cohen J. Endoscopic treatment compared with medical therapy for the prevention of recurrent ulcer hemorrhage in patients with adherent clots. Gastrointest Endosc. 2003; 58:707–714. PMID: 14595306.

32. Chiu PW, Lam CY, Lee SW, et al. Effect of scheduled second therapeutic endoscopy on peptic ulcer rebleeding: a prospective randomised trial. Gut. 2003; 52:1403–1407. PMID: 12970130.

33. Ang D, Teo EK, Tan A, et al. A comparison of surgery versus transcatheter angiographic embolization in the treatment of nonvariceal upper gastrointestinal bleeding uncontrolled by endoscopy. Eur J Gastroenterol Hepatol. 2012; 24:929–938. PMID: 22617363.

34. Wong TC, Wong KT, Chiu PW, et al. A comparison of angiographic embolization with surgery after failed endoscopic hemostasis to bleeding peptic ulcers. Gastrointest Endosc. 2011; 73:900–908. PMID: 21288512.

35. Eriksson LG, Ljungdahl M, Sundbom M, Nyman R. Transcatheter arterial embolization versus surgery in the treatment of upper gastrointestinal bleeding after therapeutic endoscopy failure. J Vasc Interv Radiol. 2008; 19:1413–1418. PMID: 18755604.

36. Ripoll C, Bañares R, Beceiro I, et al. Comparison of transcatheter arterial embolization and surgery for treatment of bleeding peptic ulcer after endoscopic treatment failure. J Vasc Interv Radiol. 2004; 15:447–450. PMID: 15126653.

37. Venclauskas L, Bratlie SO, Zachrisson K, Maleckas A, Pundzius J, Jönson C. Is transcatheter arterial embolization a safer alternative than surgery when endoscopic therapy fails in bleeding duodenal ulcer. Scand J Gastroenterol. 2010; 45:299–304. PMID: 20017710.

38. Lau JY, Sung JJ, Lam YH, et al. Endoscopic retreatment compared with surgery in patients with recurrent bleeding after initial endoscopic control of bleeding ulcers. N Engl J Med. 1999; 340:751–756. PMID: 10072409.

Fig. 1
Endoscopic findings. (A) Oozing by gastric ulcer was observed at distal antrum. (B) Oozing was stopped after injection of 1:10,000 diluted epinephrine solution.
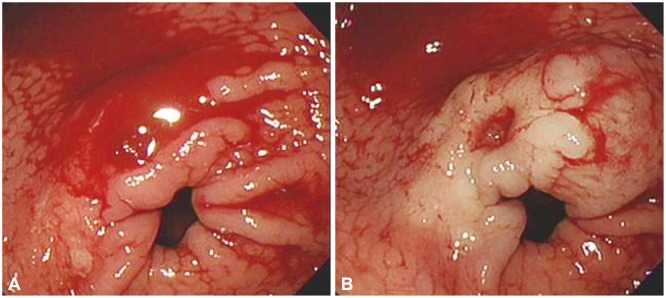
Fig. 2
Endoscopic findings. (A) A non-bleeding visible vessel on ulcer base was observed at upper body of stomach. (B) Argon plasma coagulation was performed in the visible vessel.
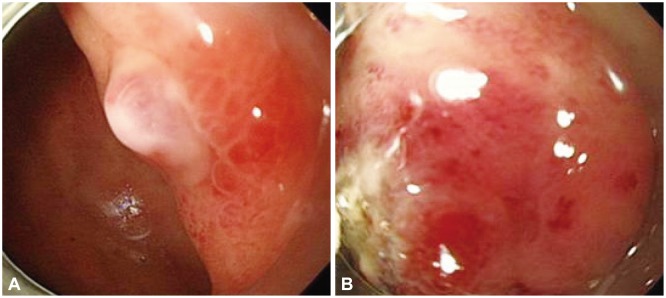
Fig. 3
Endoscopic findings. (A) A spurting was observed at duodenal ulcer. (B) A spurting was stopped by apply of endoclip.
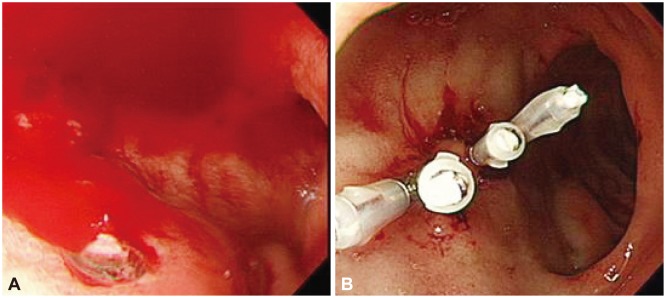
Table 1
Results of Meta-Analyses for Endoscopic Treatment Methods on Bleeding Ulcers21
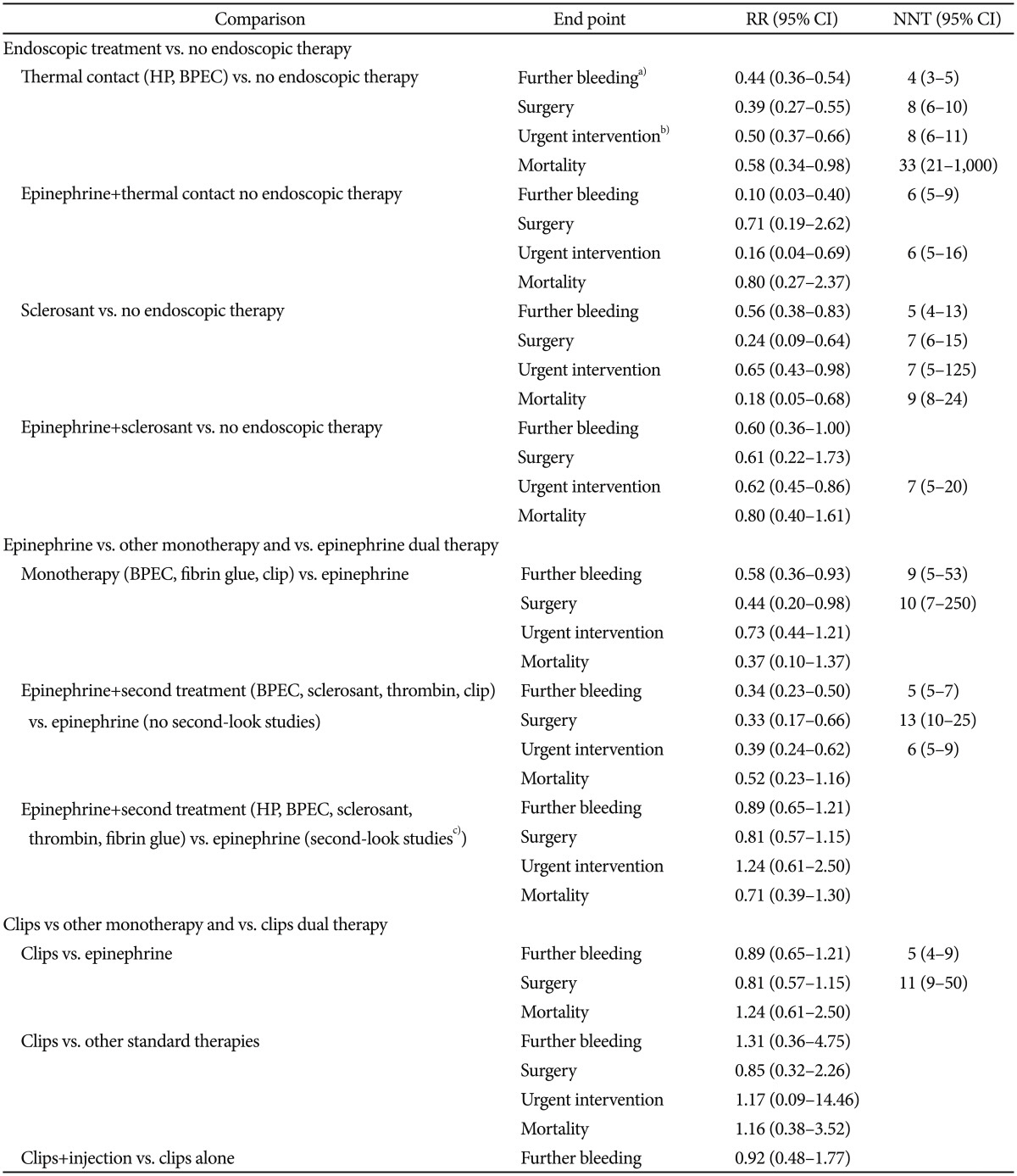
RR, relative risk; CI, confidence interval; NNT, needed number to treat; HP, heater probe; BPEC, bipolar electrocoagulation.
a)Including persistent bleeding and recurrent bleeding; b)Including subsequent endoscopic treatment with the same or different therapy, surgery or interventional radiology; c)Endoscopy that re-treatment was allowed or provided.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download