Abstract
Endometriosis is a disease characterized by the presence of endometrial tissue outside of the uterine cavity. It is common in women of childbearing age, and is most frequently located in the pelvic cavity. Approximately 10% of endometriosis cases occur outside of the pelvic cavity in locations such as the intestines, genitourinary system, kidneys, lungs, and skin. However, there have been few reports of endometriosis in the stomach. Here, we report a rare case of endometriosis that presented as a subepithelial stomach tumor.
Endometriosis is characterized by the presence of endometrial glands and stroma in an ectopic location outside of the endometrial cavity. This medical condition occurs in approximately 10% of women of reproductive age. In general, endometriosis occurs inside the pelvic cavity; however, endometriosis appears outside of the pelvic cavity in approximately 10% of all cases. Frequent locations for endometriosis outside of the pelvic cavity include a variety of tissues and organs, such as the intestines, kidneys, lungs, skin, and pleura, with the exception of the spleen.1 Endometriosis affects the gastrointestinal tract in 5% of cases, with the sigmoid colon being the most commonly affected location, followed by the rectum.2,3
However, to the best of our knowledge, the present case is among the very few reports of gastric endometriosis. Here, we report a very rare case of gastric endometriosis that presented as a subepithelial tumor.
A 44-year-old woman was referred to our institution for an asymptomatic gastric mass found during a routine check-up. A few days before her visit, the gastric mass was detected in another hospital. Her medical and family histories were unremarkable. At the time of the initial visit, she had a body temperature of 36.5℃, a pulse rate of 82 beats per minute, a respiratory rate of 18 breaths per minute, and her blood pressure was 110/80 mm Hg.
The physical examination revealed no significant abnormalities. Additionally, no remarkable findings were detected on chest or plain abdomen radiographs. Laboratory tests at admission showed a white blood cell count of 3,940/mm3 (range, 4,800 to 10,800), a hemoglobin level of 13.2 g/dL (range, 12 to 16), a carcinoembryonic antigen level of 1.35 ng/mL (range, 0 to 5), and a cancer antigen 19-9 level of 5.43 U/mL (range, 0 to 36).
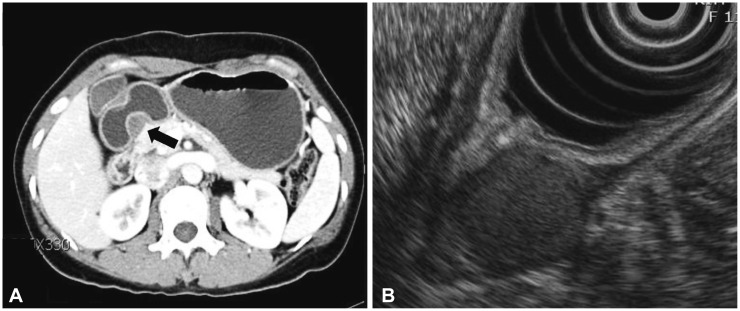
A subepithelial tumor was observed through gastroduodenoscopy on the lesser curvature of the antrum of the stomach. The lesion appeared to be 20 mm in size and was well defined. Cushion was negative (Fig. 1). A computed tomography (CT) of the abdomen showed an approximately 2-cm sized, endophytic, homogeneous, and hypodense submucosal mass in the posterior wall of the stomach antrum (Fig. 2A).
Endoscopic ultrasonography (EUS) with a radial endoscope (UE 260; Olympus, Tokyo, Japan) was performed subsequently to assess the gastric wall and evaluate the characteristics of the mass. The EUS showed a homogenous hypoechoic mass thought to have developed in the fourth hypoechoic layer (the muscularis propria); however, its echogenicity was slightly more hyperechoic than that of the proper muscle layer (Fig. 2B). It had an irregular margin and some hyperechoic foci, but did not show anechoic or hypoechoic crescent-shaped lesions. The mucosal and submucosal layers were intact. Therefore, an operable gastrointestinal stromal tumor (GIST) was suspected.
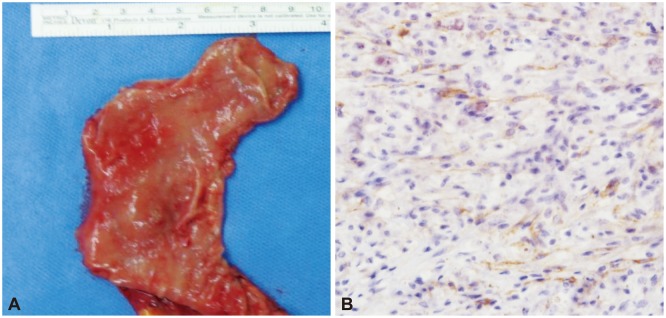
Following the EUS, laparoscopic-assisted distal gastrectomy was performed. The surgical specimen was 2.3×2.2 cm (Fig. 3A). The histopathological evaluation revealed a focus of endometriosis containing endometrial glands and hemosiderin-laden macrophages within the muscular layer near the intramural hemorrhagic cyst (Fig. 3B). This feature is compatible with endometriosis.
After the surgery, the patient was followed-up on an outpatient basis for approximately 2 years without any evidence of abnormal structure on endoscopy.
Endometriosis, a medical condition characterized by endometrial tissue outside the uterine cavity, affects approximately 10% of women, typically during childbearing age.1 Although its pathogenesis remains unclear, several theories including transplantation, coelomic metaplasia, and vascular metaplasia have been proposed.4,5 Transplantation refers to the deposition of endometrial fragments outside the uterine cavity by means of retrograde menstruation. Coelomic metaplasia could be caused by inflammation and hormonal influences such as hyperestrogenia. Additionally, the theory of coelomic metaplasia-based pathology may explain endometriosis in women with an absent uterus due to müllerian agenesis, and may also explain endometriosis in men. The theory of vascular metaplasia could explain extrapelvic endometriosis via the implantation of endometrial tissue attributable to endometrial tissue emboli disseminated through blood or lymphatic vessels.6,7
The present case represents a very rare case of gastric endometriosis. Although we could not find the exact cause of the disease, we believe coelomic metaplasia and implantation of endometrial embolism were the most likely causes of gastric endometriosis. Additionally, several authors have proposed a multifactorial origin, which led us to consider that several factors could have affected the development of gastric endometriosis simultaneously.
Gastrointestinal endometriosis is uncommon and usually asymptomatic, but may manifest as abdominal pain, pelvic pain, constipation, obstruction, or intussusceptions, among others.8,9 It usually occurs in the sigmoid colon and rectum, with small bowel endometriosis being less common (accounting for 1% to 7% of gastrointestinal endometriosis cases). Conversely, endometriosis in the stomach is considered very rare.
Kaufman et al.8 studied 89 patients with symptomatic intestinal endometriosis. Of these, 15 had endometriosis of the small bowel or proximal colon, while 74 had endometriosis of the distal colon. In their study, the most common symptom was abdominal pain. Endoscopy with mucosal biopsy led to the correct diagnosis in only 29.6% of cases, while EUS-guided biopsy led to the correct diagnosis in all four cases. EUS was performed in five patients in order to evaluate the lesion; all cases presented hypoechoic lesions, and 60% of these were inhomogeneous. Additionally, 60% of the cases showed grossly normal mucosa.
In many cases, intestinal endometriosis assumes the form of a submucosal tumor, which explains the frequent performance of EUS. On EUS, bowel endometriosis shows heterogeneous or hypoechoic crescent-shaped lesions.8,10,11,12 The heterogeneous echo finding is attributed to the presence of chocolate cysts, which result from hemorrhage of the endometriosis foci induced by the hormonal cycle.11 In previous reports, most EUS findings of bowel endometriosis showed inhomogenous and hypoechoic lesions as well.
Gastric endometriosis is very rare and has seldom been reported. Additionally, the patient in the present case had none of the typical symptoms of endometriosis, such as cyclic abdominal pain in accordance with menstruation. Therefore, we did not suspect endometriosis as a part of the differential diagnosis, and failed to obtain preoperative confirmation of gastric endometriosis.
EUS, contrast enhanced CT, and EUS-guided fine needle aspiration (EUS-FNA) have been recommended for subepithelial tumors between 2 and 5 cm.13 According to Mekky et al.,14 the diagnostic accuracy of EUS-FNA in GIST is approximately 60%. We also recommend histologic confirmation, such as EUS-FNA or incision biopsy, before surgery. However, the patient in the present case insisted on undergoing a definite surgical resection without histologic confirmation based on a discussion of the diagnostic accuracy associated with EUS-FNA and the complications associated with incision biopsy, such as severe bleeding. Because of the patient's refusal of EUS-FNA or incision biopsy, as well as our suspicion of GIST, surgical resection was performed without preoperative histologic confirmation.
Recently, another case of gastric endometriosis was reported by Kashyap et al.15 The patient was a 35-year-old woman who presented with epigastric pain, nausea, vomiting, and melena. Upper gastrointestinal endoscopy revealed a large submucosal tumor with a 1-cm ulceration on its surface. Additionally, EUS revealed a 6×6 cm, inhomogenous mass with irregular outer borders. Surgical pathology confirmed endometriosis through the presence of endometrial glands and stroma.
In cases of extrapelvic endometriosis, surgical resection with safety margins of at least 1 cm is considered the treatment of choice.16 According to the results of a previous study, surgical resection may result in a complete cure in more than 95% of patients. Furthermore, the recurrence rate after surgery was 4.3%. In the study of symptomatic intestinal endometriosis by Kaufman et al.,8 nine of the 83 patients who underwent surgical resection (10.8%) had suspected recurrence within a few years. The patient in the present case had not shown any evidence of recurrence to date, although prolonged observation was considered necessary.
The infrequent reports of gastric endometriosis make it difficult to formulate strategies for its differential diagnosis and treatment. However, we can glean some insights based on the present case and another reported case of gastric endometriosis. The EUS findings from these two gastric endometriosis cases showed a hypoechoic mass with irregular margins. The echogenicity observed in the current case was re-latively, but not completely, homogenous, and that of the other case was inhomogenous. Therefore, we can posit that the EUS finding of gastric endometriosis may present as a hypoechoic mass with inhomogenous echogenicity, or the echogenic foci characteristic of intestinal endometriosis. The treatment of choice for extrapelvic endometriosis is surgical resection with an appropriate safety margin. This approach was used in the present case, and the patient had not shown recurrence after 3 years of follow-up. Although prolonged observation is necessary, we agree that surgical resection should be considered as the primary treatment option.
In summary, gastric endometriosis presenting as a subepithelial tumor is exceptionally rare. Therefore, we reported this interesting case of gastric endometriosis initially suspected as a subepithelial tumor in order to add to the small but existing body of evidence of such occurrences.
References
1. Fernández Vozmediano JM, Armario Hita JC, Cuevas Santos J. Cutaneous endometriosis. Int J Dermatol. 2010; 49:1410–1412. PMID: 21091675.

2. Kanthimathinathan V, Elakkary E, Bleibel W, Kuwajerwala N, Conjeevaram S, Tootla F. Endometrioma of the large bowel. Dig Dis Sci. 2007; 52:767–769. PMID: 17268828.

3. Clement PB. Diseases of the peritoneum. In : Kurman RJ, editor. Blaustein's Pathology of the Female Genital Tract. 5th ed. New York: Springer-Verlag;2002. p. 761–762.
4. Ridley JH. The Histogenesis of endometriosis: a review of facts and fancies. Obstet Gynecol Surv. 1968; 23:1–35.
6. Douglas C, Rotimi O. Extragenital endometriosis: a clinicopathological review of a Glasgow hospital experience with case illustrations. J Obstet Gynaecol. 2004; 24:804–808. PMID: 15763794.
7. Veeraswamy A, Lewis M, Mann A, Kotikela S, Hajhosseini B, Nezhat C. Extragenital endometriosis. Clin Obstet Gynecol. 2010; 53:449–466. PMID: 20436322.

8. Kaufman LC, Smyrk TC, Levy MJ, Enders FT, Oxentenko AS. Symptomatic intestinal endometriosis requiring surgical resection: clinical presentation and preoperative diagnosis. Am J Gastroenterol. 2011; 106:1325–1332. PMID: 21502995.

9. Griffin JB, Betsill WL Jr. Subcutaneous endometriosis diagnosed by fine needle aspiration cytology. Acta Cytol. 1985; 29:584–588. PMID: 3861050.
10. Delpy R, Barthet M, Gasmi M, et al. Value of endorectal ultrasonography for diagnosing rectovaginal septal endometriosis infiltrating the rectum. Endoscopy. 2005; 37:357–361. PMID: 15824947.

11. Pishvaian AC, Ahlawat SK, Garvin D, Haddad NG. Role of EUS and EUS-guided FNA in the diagnosis of symptomatic rectosigmoid endometriosis. Gastrointest Endosc. 2006; 63:331–335. PMID: 16427951.

12. Chung SK, Lee SH, Son BS, et al. Rectal endometriosis that is difficult to differentiate from endoscopically resectable subepitherial lesion. Korean J Gastrointest Endosc. 2010; 41:319–323.
13. Nishida T, Kawai N, Yamaguchi S, Nishida Y. Submucosal tumors: comprehensive guide for the diagnosis and therapy of gastrointestinal submucosal tumors. Dig Endosc. 2013; 25:479–489. PMID: 23902569.

14. Mekky MA, Yamao K, Sawaki A, et al. Diagnostic utility of EUS-guided FNA in patients with gastric submucosal tumors. Gastrointest Endosc. 2010; 71:913–919. PMID: 20226456.

15. Kashyap P, Medeiros F, Levy M, Larson M. Unusual submucosal tumor in the stomach. Diagnosis: endometriosis. Gastroenterology. 2011; 140:e7–e8. PMID: 21530527.
16. Blanco RG, Parithivel VS, Shah AK, Gumbs MA, Schein M, Gerst PH. Abdominal wall endometriomas. Am J Surg. 2003; 185:596–598. PMID: 12781893.

Fig. 1
An endoscopic finding showing a 2-cm sized subepithelial tumor on the lesser curvature of the antrum. The mucosa of the lesion appears to be normal.
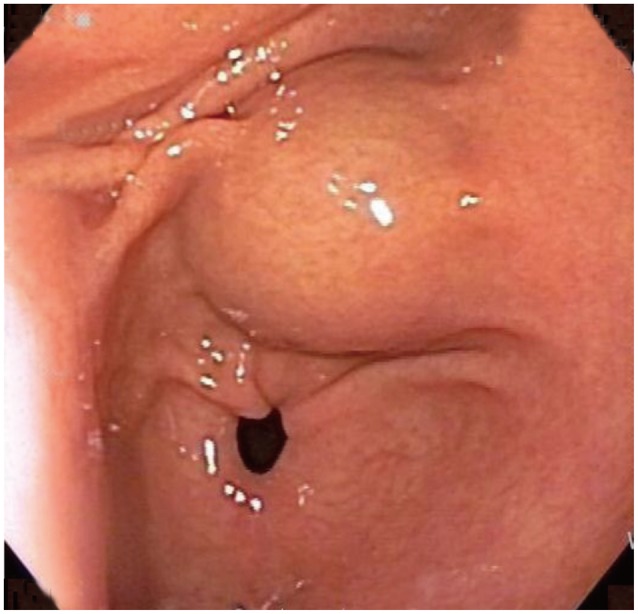
Fig. 2
(A) Abdominal computed tomography shows a gastric subepithelial tumor on the antrum (black arrow). The subepithelial mass is non-enhanced, well demarcated, and appears to be homogenous and hypodense compared to the gastric mucosa. (B) Endoscopic ultrasonography shows a homogenous hypoechoic lesion within the proper muscle layer. Its echogenicity appears to be more hyperechoic than that of the muscle layer.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download