Abstract
Biliopancreatic malignancies such as cholangiocarcinoma (CCA) has notoriously been diagnosed late. As such most therapy have been palliative in nature. Cholangioscopy allows for an earlier diagnosis to be made. Brachytherapy with the insertion of catheter with iridium-132 seeds, percutaneously or through endoscopic retrograde cholangiopancreatography (ERCP) was the earliest ablative techniques used. It has been shown to have a beneficial effect only in prolonging survival. Photodynamic therapy (PDT) has also been used for several years. stenting with PDT versus stenting alone for unresectable CCA showed a marked survival benefit with the addition of PDT. However the most exciting endoscopic ablative modality appears to be intraductal radiofrequency ablation using the Habib catheter and device. Several case series have shown the effectiveness of this technique in ablating tumors. This technique is evolving and coupled with early diagnosis of CCA through cholangioscopy will allow for a curative therapy. The crux to the effective treatment of early cancerous lesions in the bile or pancreatic duct is the early diagnosis of such lesions. Effective endoscopic ablative therapy is now available with the advent of radiofrequency ablation probes that can be passed through the duodenoscope via ERCP.
Biliopancreatic malignant neoplasia is an evolving area of interest particularly in terms of treatment since it carries a high association to morbidity and mortality. These types of neoplasia include ampullary adenocarcinoma, cholangiocarcinoma, gallbladder polyp, gallbladder cancer and pancreatic malignancies, of which pancreatic adenocarcinoma being the most common.1 Among them, cholangiocarcinoma (CCA) has the highest incidence reported in Eastern and Southeastern Asia. The main risk factor of CCA in Asian countries is mostly link to certain liver fluke infestation. Opisthorchis viverrini and Clonorchis sinensis have been associated with the development of CCA.2,3 During the early course of the disease, it runs a silent clinical course and therefore has notoriously been diagnosed late. CCA is slow growing neoplasm and is usually detected advanced at the time of diagnosis. This feature confers a grim prognosis, as such most therapy of this type of neoplasm including endoscopic therapy has always been palliative in nature. Surgical resection offers the best curative treatment, but most of these patients are noted to be unresectable and are poor surgical candidates during the time of presentation. A prospective study by Mihalache et al.4 presented the 1-year overall survival of CCA was 22.3%±4.4% and the 2-year survival was 3.4%±2.1%. They are difficult to treat and pose a dreadful outcome when inoperable. On a positive note, newer methods of direct cholangioscopy can perhaps afford an earlier diagnosis of CCA as well as lesions in the pancreatic duct and therefore allow possible "curative" endoscopic ablative therapy. Thus, different endoscopic ablative modalities are currently on investigation to achieve this therapeutic goal.
When different ablative techniques introduced into the field of gastroenterology, various diseases were addressed. These techniques can be performed directly (e.g., brachytherapy and radiofrequency ablation) or indirectly (e.g., photodynamic therapy). The route of administration is done either endoscopically or by percutaneous transhepatic cholangiography. However, such modalities are frequently employed on advanced disease stage mainly on biliopancreatic malignancies. The aim of this treatment is to ensure palliation of cholestasis or improve duration of survival and quality of life. Its use on early cancerous lesion, on the other hand however, may potentially be curative in nature. However its use remains limited due to limited evidence and further trials are recommended.
Intraluminal brachytherapy (ILBT) in biliopancreatic malignancies is one of the ablative therapies that can be performed either endoscopically or percutaneously. This modality involves the application of iridium-192 isotope seeds mounted on a catheter that are positioned directly across the area of malignant stricture in the biliary system. A variety of radiation dose between 10.4 and 20 Gy is then administered. A central feature of this therapy is that irradiation affects only the localized area around the radiation source. Hence, this technique exerts a local control of tumor by preventing tumor growth or advancement into biliary tree. This also delays the fatal complications from progressive obstructive jaundice. It can be used in conjunction with external beam radiotherapy (EBRT). The principle behind this dual system is that close proximity of the radiation source to the tumor allows administration of higher doses of radiation than what it can be achieved with EBRT alone without damaging surrounding organs.5
The evaluation of ILBT had yielded mixed results. One of the earliest endoscopic ablative therapy that utilized brachytherapy in biliopancreatic carcinoma with the insertion of catheter with iridium-192 seeds implanted either percutaneously or through endoscopic retrograde cholangiopancreatography (ERCP) as described by Montemaggi et al.6 had shown beneficial effect in prolonging survival with median survival time of 14 months in extrahepatic bile duct carcinoma and 11.5 months in pancreatic head carcinoma. Both group yielded satisfactory control of jaundice.6 In a clinical practice study of Deodato et al.,7 the application of EBRT and ILBT boost indicated an apparent survival improvement (median survival, 23 months; 1-year survival, 68%) over historic controls (median survival, 2 to 6 months; 1-year survival, 9%). However, increased late toxicity such as gastrointestinal ulcerations and mucosal erosions were observed in patients receiving brachytherapy boost.7 In a case series study involving 93 patients, the evaluation of combined-modality therapy namely ERBT, intraluminal-192 iridium and biliary stenting for unresectable extrahepatic bile duct carcinoma provided reasonable local control and improved quality of life in patients with a 1, 2, 3, and 5-year median survival rate at 49.5%, 15.1%, 9.7%, and 4.3% respectively, and a median survival period of 11.9 months.8 Another retrospective analytical study by Shin et al.9 compared two groups who underwent EBRT alone and EBRT in combination with high-dose-rate ILBT among inoperable extrahepatic bile duct carcinoma. This study suggested that high dose ILBT (total dose Gy of 15 in three fractions) was relatively safe without increased risk of radiation complications but rather most complications (e.g., duodenitis and cholangitis) were strongly related to either EBRT or insertion of the drain tubes. Likewise, addition of high-dose ILBT offered better survival benefit with a median survival time of 9 months and a 2-year survival rate of 21% when compared to EBRT alone.9
Photodynamic therapy (PDT) has been used also for several years. It is becoming one of the emerging modalities in treating CCA in patients that present with unresectable tumor. The underlying principle of PDT involves the intravenous administration of a photosensitizer which is known to accumulate preferentially in neoplastic cells, then followed by exposure of the target tissue to a light of appropriate photoactivating wavelength. This mechanism in turn commence a cascade of events by photochemical reaction to generate cytotoxic reactive oxygen species resulting in ischemia, apoptosis and necrosis of tumor cells.5,10 The complications associated with PDT are phototoxicity of the skin, increased risk of bacterial cholangitis, liver abscess, and rare cases of hemobilia.10 Hence this minimally invasive therapy is usually safe and tolerable with low complication rates.
Several studies showed favorable outcomes with the utility of this modality. Among them, the first randomized prospective controlled trial published by Ortner et al.11 in 2003 comparing PDT in addition to stenting versus stenting alone for unresectable CCA showed a marked benefit in survival, improvement of biliary drainage and quality of life with the addition of PDT. Median survival of stented patients with subsequent PDT was prolonged (median survival of 493 days) compared to stenting only (median survival of 98 days).11 Another esteemed study from Korea by Cheon et al.12 presented the advantages of PDT in a retrospective analysis of 232 patients with hilar CCA. This study revealed that PDT with stenting resulted in longer survival (median survival was 9.8 months) than stenting alone (7.3 months). They also identified several factors that may predict survival. These included lower pre-PDT bilirubin level, multiple PDT treatments and shorter time to treatment after diagnosis which were significant predictors of improved survival. On the other hand, biliary drainage without PDT and higher T-stage were significant predictors of shorter survival. Additionally, the patency of metal stent appeared to be longer in those who received PDT with median stent patency of 215 days compared to 181 days in those without PDT.12 The over-all effectiveness of PDT was determined by a systematic meta-analysis review by Legget et al.13 that yielded statistically significant trend towards benefit in terms of increased survival benefit, improved biliary drainage and quality of life. However, the quality of this evidence was low due to scarcity of published randomized controlled trials.13
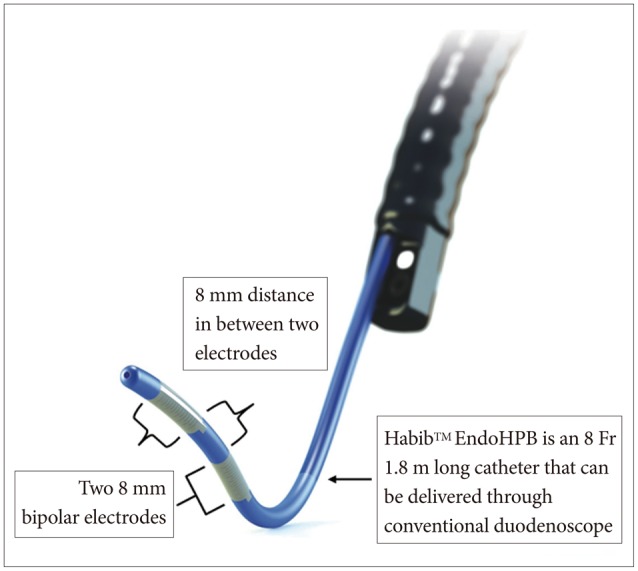
Endobiliary radiofrequency ablation (RFA) seems to be the most exciting endoscopic ablative modality using the Habib catheter and device.14,15 This system delivers both temperature-dependent and time-dependent energy deposition into bile duct tumor which thereby induces thermal injury and subsequent localized necrosis.16 Seen in Figs. 1, 2 are pre- and post-RFA cholangioscopic images courtesy of Dr. Nageshwar Reddy (Figs. 1, 2). Technically, this modality is easy to perform and can be done via percutaneous route or ERCP procedure. Endoscopically, a wire-guided Habib EndoHPB (Emcision, London, UK) catheter is placed under fluoroscopic guidance across the stricture. This catheter has a bipolar RFA probe with two ring electrodes 8 mm apart and 5 mm away from the leading edge (Fig. 3).17 The probe is 8 Fr in diameter, 1.8 m long and was shown in animal study to produce cylindrical-shaped coagulative necrosis over 2.5 cm in length. The system works using RFA generator (1500 RF generator; RITA Medical Systems, Fremont, CA, USA) delivering electrical energy at 400 kHz set at 5 to 10 W for 1 to 2 minutes.
The technical feasibility, cholangioscopic video recording and fluoroscopic images of the procedure were described in a concise manner in a pilot study conducted by Monga et al.18 Subsequent studies described by Dolak et al.,14 Steel et al.,15 Alis et al.,19 Figueroa-Barojas et al.,20 Mizandari et al.,21 and Tal et al.22 confirmed that RFA is generally a safe and feasible procedure with 100% technical success rate in most of the studies (Table 1). Several mild procedure-related complications were described by the above studies with mild postprocedure pain being the commonest. Others were pancreatitis, cholecystitis, gallbladder empyema, cholangitis but the most serious complications were hemobilia which was seen in six patients and liver infarction which was observed in one patient (Table 1).14,15,19,20,21,22
In the group with hemobilia reported by Tal et al.,22 two of the involved patients died from hemorrhagic shock while one survived after achieving hemostasis following self-expandable metallic stent (SEMS) deployment. In this group with hemobilia, it is of interest to note that plastic endoprostheses were used per protocol following RFA intervention while similar complications were not often seen in other studies that mainly utilize SEMS following RFA. It can be speculated that the stronger radial force generated by the deployment of SEMS may exert tamponade effect and facilitate hemostasis following thermal injury induced by RFA.22 Another possible explanation was the 1-minute delay in removing the RFA probe following ablation as described by Dolak et al.14 in order to avoid tissue adhesion to the heated electrodes which might cause further tissue or vascular injury upon withdrawal that may lead to hemobilia.
In the group with liver infarction, thermal injury to surrounding vessel was postulated to be the cause of liver infarction. However, the patient survived with conservative management. Liver infarction following endobiliary RFA is rare but calls for more careful pre-interventional imaging to analyze the surrounding tissue, especially vascular and biliary structures prior to RFA procedure.14
Several case series have shown the effectiveness of this technique in ablating tumors from the intraluminal ductal aspect. In a retrospective study by Dolak et al.,14 the utility of RFA in malignant biliary obstruction is found to be technically feasible, a safe therapeutic option for the palliation and yielded significant improvement in stent patency with median stent patency of 218 days in self-expandable metallic stent and 115 days in plastic stent. There was also improvement in survival benefit with overall median survival of 17.9 and 10.6 months following diagnosis and intervention respectively.14 This technique is evolving with further improvement and if coupled with early diagnosis of CCA may allow a curative therapy. However, more randomized controlled trials are needed to compare its benefit against other combination treatment such as the already proven PDT with stenting.
High-intensity intraductal ultrasound is a novel application of high-intensity ultrasound used to allow the ultrasound waves to deeply penetrate surrounding tissue and produce localized ablation of tumor cells. It is performed by passing an ultrasound probe over a guidewire into the bile duct during ERCP. Results of some studies show promising outcome in biliary tract malignancy in terms of reduction in the length of the stricture, reduction in tumor size determined by endoscopic ultrasound and the absence of malignant cells or high-grade dysplasia on cytologic specimens.5,20,22
The crux to the effective treatment of early cancerous lesions in the bile or pancreatic ducts is the early diagnosis of such lesions which has erstwhile not been possible. Most of the studies presented in this review dealt on the advantages of ablative therapies, but predominantly on its use for inoperable biliopancreatic cancer (Table 1). The ideal goal is to achieve complete ablation of such tumors and innovating these different modalities for future application that may achieve an absolute cure; thereby reducing its notorious outcome in terms of morbidity and mortality. Effective endoscopic ablative therapy is now available with the advent of radiofrequency probes that can be passed through the duodenoscope via ERCP.
References
1. American Society for Gastrointestinal Endoscopy (ASGE) Standards of Practice Committee. Anderson MA, Appalaneni V, et al. The role of endoscopy in the evaluation and treatment of patients with biliary neoplasia. Gastrointest Endosc. 2013; 77:167–174. PMID: 23219047.

2. Rerknimitr R, Angsuwatcharakon P, Ratanachu-ek T, et al. Asia-Pacific consensus recommendations for endoscopic and interventional management of hilar cholangiocarcinoma. J Gastroenterol Hepatol. 2013; 28:593–607. PMID: 23350673.

3. Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013; 145:1215–1229. PMID: 24140396.

4. Mihalache F, Tantau M, Diaconu B, Acalovschi M. Survival and quality of life of cholangiocarcinoma patients: a prospective study over a 4 year period. J Gastrointestin Liver Dis. 2010; 19:285–290. PMID: 20922193.
5. Chahal P, Baron TH. Endoscopic palliation of cholangiocarcinoma. Curr Opin Gastroenterol. 2006; 22:551–560. PMID: 16891889.

6. Montemaggi P, Costamagna G, Dobelbower RR, et al. Intraluminal brachytherapy in the treatment of pancreas and bile duct carcinoma. Int J Radiat Oncol Biol Phys. 1995; 32:437–443. PMID: 7538501.

7. Deodato F, Clemente G, Mattiucci GC, et al. Chemoradiation and brachytherapy in biliary tract carcinoma: long-term results. Int J Radiat Oncol Biol Phys. 2006; 64:483–488. PMID: 16242254.

8. Takamura A, Saito H, Kamada T, et al. Intraluminal low-dose-rate 192Ir brachytherapy combined with external beam radiotherapy and biliary stenting for unresectable extrahepatic bile duct carcinoma. Int J Radiat Oncol Biol Phys. 2003; 57:1357–1365. PMID: 14630274.

9. Shin HS, Seong J, Kim WC, et al. Combination of external beam irradiation and high-dose-rate intraluminal brachytherapy for inoperable carcinoma of the extrahepatic bile ducts. Int J Radiat Oncol Biol Phys. 2003; 57:105–112. PMID: 12909222.

10. Charbel H, Al-Kawas FH. Cholangiocarcinoma treatment. Curr Gastroenterol Rep. 2012; 14:528–533. PMID: 22968375.

11. Ortner ME, Caca K, Berr F, et al. Successful photodynamic therapy for nonresectable cholangiocarcinoma: a randomized prospective study. Gastroenterology. 2003; 125:1355–1363. PMID: 14598251.

12. Cheon YK, Lee TY, Lee SM, Yoon JY, Shim CS. Longterm outcome of photodynamic therapy compared with biliary stenting alone in patients with advanced hilar cholangiocarcinoma. HPB (Oxford). 2012; 14:185–193. PMID: 22321037.

13. Leggett CL, Gorospe EC, Murad MH, Montori VM, Baron TH, Wang KK. Photodynamic therapy for unresectable cholangiocarcinoma: a comparative effectiveness systematic review and meta-analyses. Photodiagnosis Photodyn Ther. 2012; 9:189–195. PMID: 22959798.

14. Dolak W, Schreiber F, Schwaighofer H, et al. Endoscopic radiofrequency ablation for malignant biliary obstruction: a nationwide retrospective study of 84 consecutive applications. Surg Endosc. 2014; 28:854–860. PMID: 24196547.

15. Steel AW, Postgate AJ, Khorsandi S, et al. Endoscopically applied radiofrequency ablation appears to be safe in the treatment of malignant biliary obstruction. Gastrointest Endosc. 2011; 73:149–153. PMID: 21184881.

16. Tatli S, Tapan U, Morrison PR, Silverman SG. Radiofrequency ablation: technique and clinical applications. Diagn Interv Radiol. 2012; 18:508–516. PMID: 22407695.

17. EMcision. Habib EndoHPB catheter [Internet]. Montreal: EMcision;2014. cited 2015 Jan 12. Available from: http://emcision.com/products/habib-endohpb1/.
18. Monga A, Gupta R, Ramchandani M, Rao GV, Santosh D, Reddy DN. Endoscopic radiofrequency ablation of cholangiocarcinoma: new palliative treatment modality (with videos). Gastrointest Endosc. 2011; 74:935–937. PMID: 21168839.

19. Alis H, Sengoz C, Gonenc M, Kalayci MU, Kocatas A. Endobiliary radiofrequency ablation for malignant biliary obstruction. Hepatobiliary Pancreat Dis Int. 2013; 12:423–427. PMID: 23924501.

20. Figueroa-Barojas P, Bakhru MR, Habib NA, et al. Safety and efficacy of radiofrequency ablation in the management of unresectable bile duct and pancreatic cancer: a novel palliation technique. J Oncol. 2013; 2013:910897. PMID: 23690775.

21. Mizandari M, Pai M, Xi F, et al. Percutaneous intraductal radiofrequency ablation is a safe treatment for malignant biliary obstruction: feasibility and early results. Cardiovasc Intervent Radiol. 2013; 36:814–819. PMID: 23232859.

22. Tal AO, Vermehren J, Friedrich-Rust M, et al. Intraductal endoscopic radiofrequency ablation for the treatment of hilar non-resectable malignant bile duct obstruction. World J Gastrointest Endosc. 2014; 6:13–19. PMID: 24527176.

Fig. 1
Preradiofrequency ablation cholangioscopic image of cholangiocarcinoma (courtesy of Dr Nageshwar Reddy; personal collection).
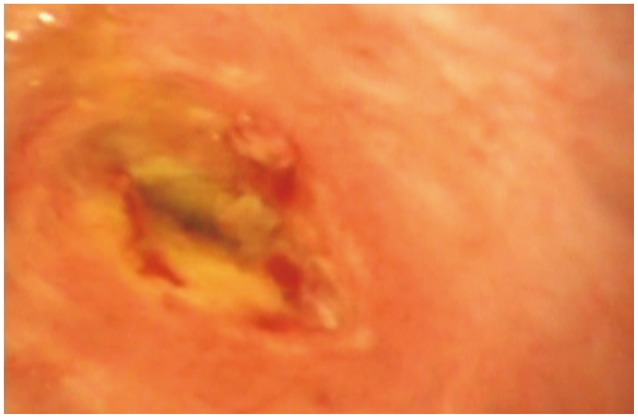
Fig. 2
Two-week postradiofrequency ablation cholangioscopic image of cholangiocarcinoma (courtesy of Dr Nageshwar Reddy; personal collection).
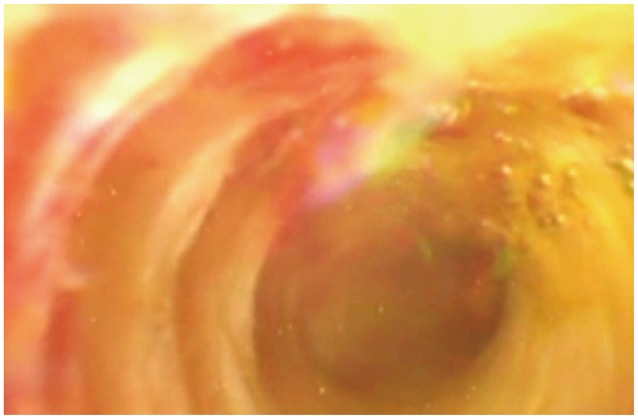
Fig. 3
Habib EndoHPB catheter is delivered through side-viewing duodenoscope. Adapted from EMcision, Habib EndoHPB catheter, with permission from EMcision.17




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download