Abstract
During the last decade, great progress has been made in minimally invasive endoscopic techniques. For pancreatic pseudocysts (PPCs), endoscopic drainage has become the first-line therapeutic option. Recent advances in therapeutic endoscopic ultrasound (EUS)-related techniques have focused on EUS-guided transmural drainage, which is now replacing the conventional endoscopy-guided transmural drainage. While transmural drainage is usually performed using multiple plastic stents with or without a nasocystic drain, fully covered self-expandable metal stents are now being used with increasing frequency. In this review, we discuss some of the controversies related to the endoscopic drainage of PPCs.
Pancreatic pseudocysts (PPCs) may develop in 10% to 20% of patients with acute pancreatitis and may be present in 20% to 40% of patients with chronic pancreatitis.1,2 During the last decade, the treatment strategies for PPCs have evolved dramatically in terms of indications, patient selection, and dedicated treatment methods.3 This progress may be attributed to the development of minimally invasive approaches. Endoscopic drainage has become the procedure of choice for the management of most patients with symptomatic PPCs, although there are a number of issues to be considered prior to the implementation of this procedure. Endoscopic ultrasound (EUS) facilitates the transmural drainage of PPCs and may increase the success rate and safety of PPC drainage. In addition, EUS techniques may enhance the endoscopic treatment of pancreatic necrosis and disconnected pancreatic duct syndrome. In this study, we review the latest developments in the endoscopic drainage of PPCs.
Although a high treatment success rate for endoscopic PPC drainage has been reported, the response depends on the type of pseudocysts.4 Therefore, PPCs must be distinguished from acute peripancreatic fluid collections, acute necrotic collections, walled-off pancreatic necrosis (WOPN), and cystic neoplasms. At a recent consensus conference, the terms pancreatic or peripancreatic fluid and necrotic collections were more systematically defined.5 Acute fluid collections occur within 4 weeks after the onset of pancreatitis; the collections do not replace pancreas parenchyma or have a well-defined wall. PPCs consist of localized fluid that are within or adjacent to the pancreas and are enclosed in a well-defined, nonepithelialized wall with either simple or complex contents. PPCs persist for more than 4 weeks after the onset of pancreatitis. PPCs may communicate with the pancreatic duct and may be associated with an underlying ductal stricture or leak.
Necrosis is a region of necrotic pancreatic parenchyma and/or peripancreatic fat. Acute necrotic collections occur within 4 weeks, whereas WOPN persists for more than 4 weeks. WOPN develops only after acute necrotizing pancreatitis and can be intrapancreatic or extrapancreatic. WOPN contains nonliquid material with varying amounts of fluid and has an encapsulating wall. Although the overall clinical success for endoscopic drainage of PPCs is high, previous trials have demonstrated that the response depends on the type of pancreatic fluid collection that develops. For instance, the treatment success for PPCs is greater than 90%, but it decreases to 50% to 65% for WOPN.6 Since the necrotic contents of WOPN are highly dense, larger fistulous openings or multiple tracts are required for effective drainage. Therefore, WOPN approaches are complex and differ from that for simple PPCs, demonstrating the crucial clinical implications of this distinction.
Finally, PPCs must be distinguished from pancreatic cystic neoplasms, which may require surgical resection.3 In patients with no history of pancreatitis, cystic neoplasms should be considered first. It should also be remembered that pancreatic cystic neoplasms may occasionally accompany a bout of acute pancreatitis.
Previous indications for the treatment of PPCs based on the size and the duration of PPCs have become obsolete.7,8 Widely accepted indications for PPC drainage include persistent abdominal symptoms (i.e., abdominal pain or early satiety), the obstruction of surrounding hollow viscous, cyst-related adverse events (i.e., biliary obstruction, bleeding, or infection), and the rapid enlargement of a cyst.2,9,10,11,12 Prior to drainage, it is usually desirable to wait until the fluid collection develops into a wall or fibrous capsule. However, if there is a pressing indication, such as a poorly controlled infection, the drainage procedure may be performed within 2 weeks of the onset of pancreatitis.13
Surgery was the gold standard treatment for PPCs for many decades, but it has now been replaced by the endoscopic drainage technique. Currently, endoscopic drainage is recommended as the first-line treatment for accessible PPCs because it can provide excellent results in terms of costs, duration of hospital stay, and quality of life, as demonstrated in a recent prospective randomized study.14 However, this trend may not be uniform, and the treatment decision may vary with local expertise. Various factors can influence the decision to proceed with endoscopic drainage, including anatomical factors such as PPC location, main pancreatic ductal anatomy, and the communication between PPCs and the pancreatic duct.
It is common practice to use endoscopic retrograde pancreatography (ERP) to address main pancreatic duct (MPD) strictures and leaks before the PPC drainage procedure is performed. A direct communication between PPCs and the MPD is present in 40% to 66% of all PPCs and may allow transpapillary drainage of the PPCs. However, the transpapillary drainage of PPCs is usually reserved for relatively small PPCs of less than 5 cm, especially those which are located in the pancreas head.15 However, in cases involving large PPCs, ERP may be very difficult or impossible owing to the severe luminal compression of the stomach or duodenum by PPCs. Transmural drainage should be performed first in this cases, following ERP, to confirm if pancreatic duct stricture or leak has resolved or if PPCs have significantly decreased. If an MPD leak is not present, the transmural stents can be removed. If an MPD leak is present, endoscopic pancreatic sphincterotomy and MPD stenting that bridge the leak site are needed, and a more long-term placement of the transmural stent is usually recommended. MPD stents are usually maintained for 4 weeks and can be removed together with the transmural stents after the resolution of the MPD leak is confirmed.
It is generally accepted that transmural stents should be kept in place until the PPCs are completely resolved and not before at least 2 months of stenting. According to previous studies, the recurrence rate of PPCs was significantly higher in the early stent retrieval group, and stenting duration of less than 6 weeks was independently associated with treatment failure.12,16
EUS-guided drainage has replaced conventional endoscopyguided drainage, in which a duodenoscope is used; this is because of the advantages of the EUS-guided technique, including the possibility of safely draining nonbulging PPCs.8 In addition to nonbulging PPCs, EUS guidance may be preferred in patients with vascular collaterals such as those with splenic vein thrombosis, coagulopathy, a small anatomic window for drainage, and those who would benefit from transduodenal rather than transgastric drainage.8,13 At many centers, EUS-guided drainage is increasingly being used and is recognized as the standard procedure for the management of symptomatic PPCs. EUS-guided drainage shows a technical success rate of more than 90% and a treatment success rate of 75% to 90%, depending on the PPC characteristics. Two randomized trials that compared EUS-guided PPC drainage and conventional endoscopy-guided PPC drainage, demonstrated that EUS-guided drainage is superior to conventional endoscopy-guided drainage in terms of technical success and occurrence of procedure-related adverse events.17,18
Despite recent advances in interventional endoscopy, many questions remain about the type, size, and number of stents that should be used for PPC drainage. To achieve adequate drainage, multiple double pigtail plastic stents are usually inserted for transmural drainage; a nasocystic drain may occasionally be left in place to irrigate the PPCs with saline if viscous debris is present. In PPCs with viscous debris, a dual drainage with transmural plastic stents and a nasocystic drain may lower the stent occlusion rate and may improve clinical outcomes compared with those that are obtained via plastic stents alone.4 The vigorous irrigation with saline removes solid debris, decreases stent occlusion rates, and improves overall clinical outcomes. However, plastic stents with small diameters may fundamentally not allow effective drainage or may result in superinfections of the PPCs, which typically occur when plastic stents are obstructed.
Recently, some studies have reported the usefulness of fully covered self-expandable metal stents (FCSEMSs) for EUS-guided drainage. The insertion of multiple stents and/or a nasocystic drain is time-consuming and may often be technically challenging.19 On the other hand, FCSEMSs are easier to deploy and preclude the need to place multiple plastic stents and therefore, seem to be a promising alternative for PPC drainage, particularly for PPCs with thick debris.20 Recent studies suggested that using FCSEMSs in patients with infected PPCs may decrease the need for repeated endoscopic procedures, increase the treatment success rate, and reduce the time required for PPC resolution.21,22,23 Diverse FCSEMSs specifically designed for PPC drainage, which have a short length, a large lumen, and a unique structure to prevent stent migration, have also become available.24,25 In addition, recently introduced metal stents have a larger diameter; therefore, these stents can be used as the port of entry for endoscopic necrosectomy.26
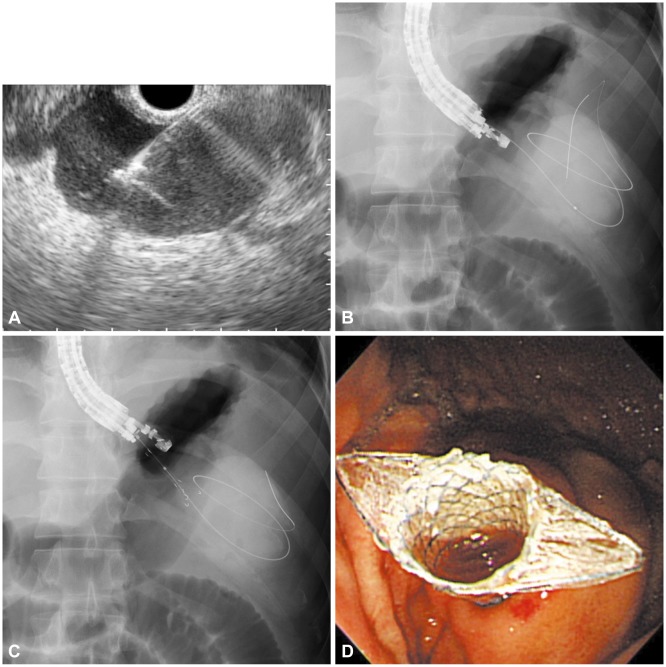
According to our experience, EUS-guided drainage with FCSEMSs, which are specially designed for PPC drainage (Fig. 1), yield similar technical success rates, clinical success rates, and adverse event rates as plastic stents, but are associated with a significantly shorter procedure time than other stents. Our experiences showed that the mean procedure time was 15 minutes in the FCSEMS group, but 29.5 minutes in the plastic stent group. The shorter procedure time in the FCSEMS group was attributed to the simpler process that requires one stent and one guidewire (Figs. 1, 2). In this manner, the insertion of multiple guidewires and stents can be avoided. Even if a nasocystic drain were needed, an additional guidewire insertion would be unnecessary. In terms of cost-effectiveness, there may be an argument because of the relatively high cost of metal stents compared to plastic stents. However, EUS-guided drainage with FCSEMSs does not require balloon dilatation and multiple guidewires; there may not be a significant difference between the costs. In addition, in our opinion, the use of FCSEMSs might be suitable for endoscopists who do not have much experience with the EUS-guided intervention due to the simplified procedure.
Adverse events related to the endoscopic drainage of PPCs largely vary among centers, with the average morbidity rates being 13% and the average mortality rates being 0.3%.27 Major adverse events reported include hemorrhage, perforation, and infection. Most of these events can be managed without surgery through approaches such as endoscopic coagulation, arterial embolization, intravenous antibiotics, or repeated endoscopic drainage in the case of secondary infection.
As the management paradigm invariably shifts to less invasive techniques, endoscopic approaches will play an increasing role in the management of PPCs. More importantly, utilizing EUS guidance for transmural drainage facilitates the drainage procedure of PPCs and may increase the success rate and safety of this approach. With the advent of FCSEMSs specifically designed for transmural drainage, these stents may become increasingly useful towards furthering the advancement of the endoscopic treatment of PPCs.
References
1. Barthet M, Bugallo M, Moreira LS, Bastid C, Sastre B, Sahel J. Treatment of pseudocysts in acute pancreatitis. Retrospective study of 45 patients. Gastroenterol Clin Biol. 1992; 16:853–859. PMID: 1483554.
2. Beckingham IJ, Krige JE, Bornman PC, Terblanche J. Endoscopic management of pancreatic pseudocysts. Br J Surg. 1997; 84:1638–1645. PMID: 9448608.

3. Chauhan SS, Forsmark CE. Evidence-based treatment of pancreatic pseudocysts. Gastroenterology. 2013; 145:511–513. PMID: 23900106.

4. Siddiqui AA, Dewitt JM, Strongin A, et al. Outcomes of EUS-guided drainage of debris-containing pancreatic pseudocysts by using combined endoprosthesis and a nasocystic drain. Gastrointest Endosc. 2013; 78:589–595. PMID: 23660566.

5. Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis: 2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013; 62:102–111. PMID: 23100216.
6. Varadarajulu S, Bang JY, Phadnis MA, Christein JD, Wilcox CM. Endoscopic transmural drainage of peripancreatic fluid collections: outcomes and predictors of treatment success in 211 consecutive patients. J Gastrointest Surg. 2011; 15:2080–2088. PMID: 21786063.

7. Andrén-Sandberg A, Dervenis C. Pancreatic pseudocysts in the 21st century. Part II: natural history. JOP. 2004; 5:64–70. PMID: 15007187.
8. Singhal S, Rotman SR, Gaidhane M, Kahaleh M. Pancreatic fluid collection drainage by endoscopic ultrasound: an update. Clin Endosc. 2013; 46:506–514. PMID: 24143313.

9. Kahaleh M, Shami VM, Conaway MR, et al. Endoscopic ultrasound drainage of pancreatic pseudocyst: a prospective comparison with conventional endoscopic drainage. Endoscopy. 2006; 38:355–359. PMID: 16680634.

10. Varadarajulu S, Wilcox CM, Tamhane A, Eloubeidi MA, Blakely J, Canon CL. Role of EUS in drainage of peripancreatic fluid collections not amenable for endoscopic transmural drainage. Gastrointest Endosc. 2007; 66:1107–1119. PMID: 17892874.

11. Jacobson BC, Baron TH, Adler DG, et al. ASGE guideline: the role of endoscopy in the diagnosis and the management of cystic lesions and inflammatory fluid collections of the pancreas. Gastrointest Endosc. 2005; 61:363–370. PMID: 15758904.

12. Cahen D, Rauws E, Fockens P, Weverling G, Huibregtse K, Bruno M. Endoscopic drainage of pancreatic pseudocysts: long-term outcome and procedural factors associated with safe and successful treatment. Endoscopy. 2005; 37:977–983. PMID: 16189770.

13. Topazian M. Endoscopic ultrasound-guided drainage of pancreatic fluid collections (with video). Clin Endosc. 2012; 45:337–340. PMID: 22977831.

14. Varadarajulu S, Bang JY, Sutton BS, Trevino JM, Christein JD, Wilcox CM. Equal efficacy of endoscopic and surgical cystogastrostomy for pancreatic pseudocyst drainage in a randomized trial. Gastroenterology. 2013; 145:583–590. PMID: 23732774.

15. Baron TH. Endoscopic drainage of pancreatic pseudocysts. J Gastrointest Surg. 2008; 12:369–372. PMID: 17906903.

16. Arvanitakis M, Delhaye M, Bali MA, et al. Pancreatic-fluid collections: a randomized controlled trial regarding stent removal after endoscopic transmural drainage. Gastrointest Endosc. 2007; 65:609–619. PMID: 17324413.

17. Park DH, Lee SS, Moon SH, et al. Endoscopic ultrasound-guided versus conventional transmural drainage for pancreatic pseudocysts: a prospective randomized trial. Endoscopy. 2009; 41:842–848. PMID: 19798610.

18. Varadarajulu S, Christein JD, Tamhane A, Drelichman ER, Wilcox CM. Prospective randomized trial comparing EUS and EGD for transmural drainage of pancreatic pseudocysts (with videos). Gastrointest Endosc. 2008; 68:1102–1111. PMID: 18640677.

19. Binmoeller KF, Weilert F, Shah JN, Bhat YM, Kane S. Endosonographyguided transmural drainage of pancreatic pseudocysts using an exchange-free access device: initial clinical experience. Surg Endosc. 2013; 27:1835–1839. PMID: 23299130.

20. Bang JY, Varadarajulu S. Metal versus plastic stent for transmural drainage of pancreatic fluid collections. Clin Endosc. 2013; 46:500–502. PMID: 24143311.

21. Gornals JB, De la Serna-Higuera C, Sànchez-Yague A, Loras C, Sànchez-Cantos AM, Pérez-Miranda M. Endosonography-guided drainage of pancreatic fluid collections with a novel lumen-apposing stent. Surg Endosc. 2013; 27:1428–1434. PMID: 23232994.

22. Talreja JP, Shami VM, Ku J, Morris TD, Ellen K, Kahaleh M. Transenteric drainage of pancreatic-fluid collections with fully covered self-expanding metallic stents (with video). Gastrointest Endosc. 2008; 68:1199–1203. PMID: 19028232.

23. Berzosa M, Maheshwari S, Patel KK, Shaib YH. Single-step endoscopic ultrasonography-guided drainage of peripancreatic fluid collections with a single self-expandable metal stent and standard linear echoendoscope. Endoscopy. 2012; 44:543–547. PMID: 22407382.

24. Yamamoto N, Isayama H, Kawakami H, et al. Preliminary report on a new, fully covered, metal stent designed for the treatment of pancreatic fluid collections. Gastrointest Endosc. 2013; 77:809–814. PMID: 23453183.

25. Itoi T, Binmoeller KF, Shah J, et al. Clinical evaluation of a novel lumenapposing metal stent for endosonography-guided pancreatic pseudocyst and gallbladder drainage (with videos). Gastrointest Endosc. 2012; 75:870–876. PMID: 22301347.

26. Antillon MR, Bechtold ML, Bartalos CR, Marshall JB. Transgastric endoscopic necrosectomy with temporary metallic esophageal stent placement for the treatment of infected pancreatic necrosis (with video). Gastrointest Endosc. 2009; 69:178–180. PMID: 18582877.

27. Dumonceau JM, Macias-Gomez C. Endoscopic management of complications of chronic pancreatitis. World J Gastroenterol. 2013; 19:7308–7315. PMID: 24259962.

Fig. 1
(A) Fluoroscopic image showing a endoscopic ultrasound-guided transgastric puncture of the pancreatic pseudocyst (PPC). (B) Coiling of the guidewire within the PPC under fluoroscopic guidance. (C) Dilation of the tract with a needle-knife. (D) Placement of a fully covered self-expandable metal stent.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download