1. Li QL, Zhang YQ, Chen WF, et al. Endoscopic submucosal dissection for foregut neuroendocrine tumors: an initial study. World J Gastroenterol. 2012; 18:5799–5806. PMID:
23155323.

2. Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010; 39:707–712. PMID:
20664470.
3. Rindi G, Arnold R, Bosman FT, et al. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. The International Agency for Research on Cancer. In : Bosman TF, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon: International Agency for Research on Cancer;2010. p. 13.
4. Hruban RH, Pitman MB, Klimstra DS. American Registry of Pathology. Armed Forces Institute of Pathology (U.S.). Tumors of the Pancreas. Washington DC: American Registry of Pathology in collaboration with the Armed Forces Institute of Pathology;2007. p. 422.
5. Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008; 26:3063–3072. PMID:
18565894.

6. Tsai HJ, Wu CC, Tsai CR, Lin SF, Chen LT, Chang JS. The epidemiology of neuroendocrine tumors in taiwan: a nation-wide cancer registry-based study. PLoS One. 2013; 8:e62487. PMID:
23614051.

7. Hauso O, Gustafsson BI, Kidd M, et al. Neuroendocrine tumor epidemiology: contrasting Norway and North America. Cancer. 2008; 113:2655–2664. PMID:
18853416.
8. Ito T, Sasano H, Tanaka M, et al. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J Gastroenterol. 2010; 45:234–243. PMID:
20058030.

9. Ellis L, Shale MJ, Coleman MP. Carcinoid tumors of the gastrointestinal tract: trends in incidence in England since 1971. Am J Gastroenterol. 2010; 105:2563–2569. PMID:
20823835.

10. Scherübl H. Options for gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008; 9:203. PMID:
18308249.

11. Hosokawa O, Miyanaga T, Kaizaki Y, et al. Decreased death from gastric cancer by endoscopic screening: association with a population-based cancer registry. Scand J Gastroenterol. 2008; 43:1112–1115. PMID:
18609154.

12. Scherübl H, Cadiot G, Jensen RT, Rösch T, Stölzel U, Klöppel G. Neuroendocrine tumors of the stomach (gastric carcinoids) are on the rise: small tumors, small problems? Endoscopy. 2010; 42:664–671. PMID:
20669078.

13. Scherübl H. Rectal carcinoids are on the rise: early detection by screening endoscopy. Endoscopy. 2009; 41:162–165. PMID:
19214898.

14. Scherübl H, Jensen RT, Cadiot G, Stölzel U, Klöppel G. Neuroendocrine tumors of the small bowels are on the rise: early aspects and management. World J Gastrointest Endosc. 2010; 2:325–334. PMID:
21160582.

15. Modlin IM, Lye KD, Kidd M. A 50-year analysis of 562 gastric carcinoids: small tumor or larger problem? Am J Gastroenterol. 2004; 99:23–32. PMID:
14687136.

16. Gencosmanoglu R, Sen-Oran E, Kurtkaya-Yapicier O, Avsar E, Sav A, Tozun N. Gastric polypoid lesions: analysis of 150 endoscopic polypectomy specimens from 91 patients. World J Gastroenterol. 2003; 9:2236–2239. PMID:
14562385.

17. Scherübl H, Jensen RT, Cadiot G, Stölzel U, Klöppel G. Management of early gastrointestinal neuroendocrine neoplasms. World J Gastrointest Endosc. 2011; 3:133–139. PMID:
21860682.

18. Zhang L, Ozao J, Warner R, Divino C. Review of the pathogenesis, diagnosis, and management of type I gastric carcinoid tumor. World J Surg. 2011; 35:1879–1886. PMID:
21559999.

19. Borch K, Ahrén B, Ahlman H, Falkmer S, Granérus G, Grimelius L. Gastric carcinoids: biologic behavior and prognosis after differentiated treatment in relation to type. Ann Surg. 2005; 242:64–73. PMID:
15973103.
20. Oberg K. Neuroendocrine gastrointestinal tumours. Ann Oncol. 1996; 7:453–463. PMID:
8839899.
21. Granberg D, Wilander E, Stridsberg M, Granerus G, Skogseid B, Oberg K. Clinical symptoms, hormone profiles, treatment, and prognosis in patients with gastric carcinoids. Gut. 1998; 43:223–228. PMID:
10189848.

22. Nobels FR, Kwekkeboom DJ, Coopmans W, et al. Chromogranin A as serum marker for neuroendocrine neoplasia: comparison with neuronspecific enolase and the alpha-subunit of glycoprotein hormones. J Clin Endocrinol Metab. 1997; 82:2622–2628. PMID:
9253344.
23. Campana D, Nori F, Pezzilli R, et al. Gastric endocrine tumors type I: treatment with long-acting somatostatin analogs. Endocr Relat Cancer. 2008; 15:337–342. PMID:
18310299.

24. Fykse V, Sandvik AK, Qvigstad G, Falkmer SE, Syversen U, Waldum HL. Treatment of ECL cell carcinoids with octreotide LAR. Scand J Gastroenterol. 2004; 39:621–628. PMID:
15370681.

25. Shi W, Johnston CF, Buchanan KD, et al. Localization of neuroendocrine tumours with [111In] DTPA-octreotide scintigraphy (Octreoscan): a comparative study with CT and MR imaging. QJM. 1998; 91:295–301. PMID:
9666953.

26. Rehm H, Wiedenmann B, Betz H. Molecular characterization of synaptophysin, a major calcium-binding protein of the synaptic vesicle membrane. EMBO J. 1986; 5:535–541. PMID:
3086086.

27. Erickson JD, Schafer MK, Bonner TI, Eiden LE, Weihe E. Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter. Proc Natl Acad Sci U S A. 1996; 93:5166–5171. PMID:
8643547.

28. Rindi G, Paolotti D, Fiocca R, Wiedenmann B, Henry JP, Solcia E. Vesicular monoamine transporter 2 as a marker of gastric enterochromaffin-like cell tumors. Virchows Arch. 2000; 436:217–223. PMID:
10782879.

29. Nikou GC, Angelopoulos TP. Current concepts on gastric carcinoid tumors. Gastroenterol Res Pract. 2012; 2012:287825. PMID:
23316222.

30. Rindi G, Bordi C, Rappel S, La Rosa S, Stolte M, Solcia E. Gastric carcinoids and neuroendocrine carcinomas: pathogenesis, pathology, and behavior. World J Surg. 1996; 20:168–172. PMID:
8661813.

31. Ravizza D, Fiori G, Trovato C, et al. Long-term endoscopic and clinical follow-up of untreated type 1 gastric neuroendocrine tumours. Dig Liver Dis. 2007; 39:537–543. PMID:
17433795.

32. Merola E, Sbrozzi-Vanni A, Panzuto F, et al. Type I gastric carcinoids: a prospective study on endoscopic management and recurrence rate. Neuroendocrinology. 2012; 95:207–213. PMID:
21811050.

33. Soga J. Early-stage carcinoids of the gastrointestinal tract: an analysis of 1914 reported cases. Cancer. 2005; 103:1587–1595. PMID:
15742328.
34. Ramage JK, Ahmed A, Ardill J, et al. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs). Gut. 2012; 61:6–32. PMID:
22052063.

35. Yamamoto C, Aoyagi K, Suekane H, et al. Carcinoid tumors of the duodenum: report of three cases treated by endoscopic resection. Endoscopy. 1997; 29:218–221. PMID:
9201476.

36. Nishimori I, Morita M, Sano S, et al. Endosonography-guided endoscopic resection of duodenal carcinoid tumor. Endoscopy. 1997; 29:214–217. PMID:
9201475.

37. Yoshikane H, Goto H, Niwa Y, et al. Endoscopic resection of small duodenal carcinoid tumors with strip biopsy technique. Gastrointest Endosc. 1998; 47:466–470. PMID:
9647370.

38. Zyromski NJ, Kendrick ML, Nagorney DM, et al. Duodenal carcinoid tumors: how aggressive should we be? J Gastrointest Surg. 2001; 5:588–593. PMID:
12086896.

39. Ichikawa J, Tanabe S, Koizumi W, et al. Endoscopic mucosal resection in the management of gastric carcinoid tumors. Endoscopy. 2003; 35:203–206. PMID:
12584637.

40. Hopper AD, Bourke MJ, Hourigan LF, Tran K, Moss A, Swan MP. Enbloc resection of multiple type 1 gastric carcinoid tumors by endoscopic multi-band mucosectomy. J Gastroenterol Hepatol. 2009; 24:1516–1521. PMID:
19743997.

41. Yokoyama S, Takifuji K, Tani M, et al. Endoscopic resection of duodenal bulb neuroendocrine tumor larger than 10 mm in diameter. BMC Gastroenterol. 2011; 11:67. PMID:
21658277.

42. Nikou GC, Toubanakis C, Moulakakis KG, et al. Carcinoid tumors of the duodenum and the ampulla of Vater: current diagnostic and therapeutic approach in a series of 8 patients. Case series. Int J Surg. 2011; 9:248–253. PMID:
21215338.

43. Kolby L, Nilsson O, Ahlman H. Gastroduodenal endocrine tumours. Scand J Surg. 2004; 93:317–323. PMID:
15658674.

44. Burke AP, Sobin LH, Federspiel BH, Shekitka KM, Helwig EB. Carcinoid tumors of the duodenum. A clinicopathologic study of 99 cases. Arch Pathol Lab Med. 1990; 114:700–704. PMID:
1694655.
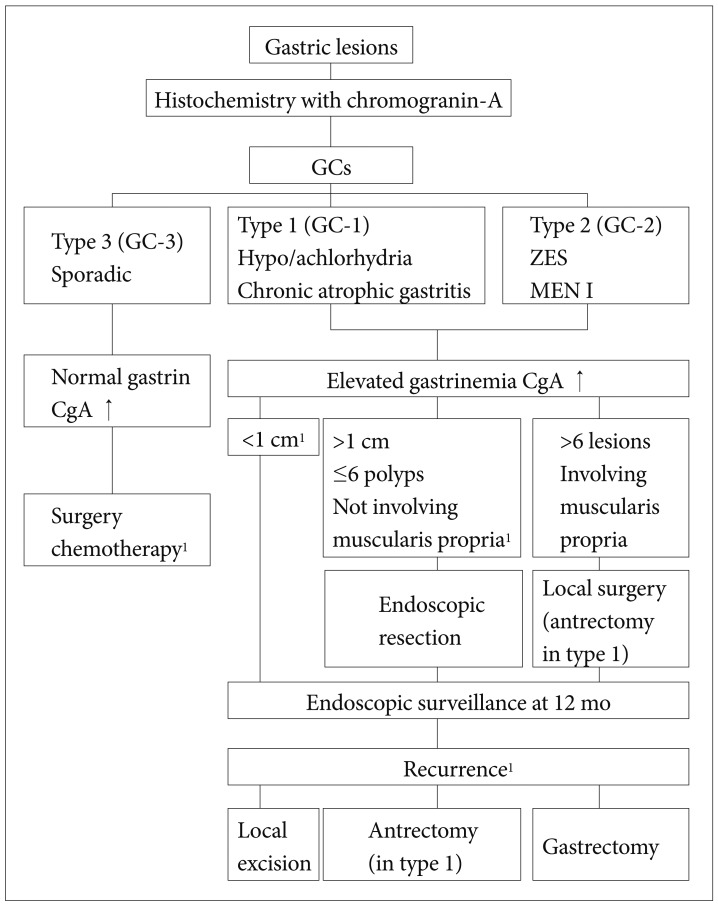
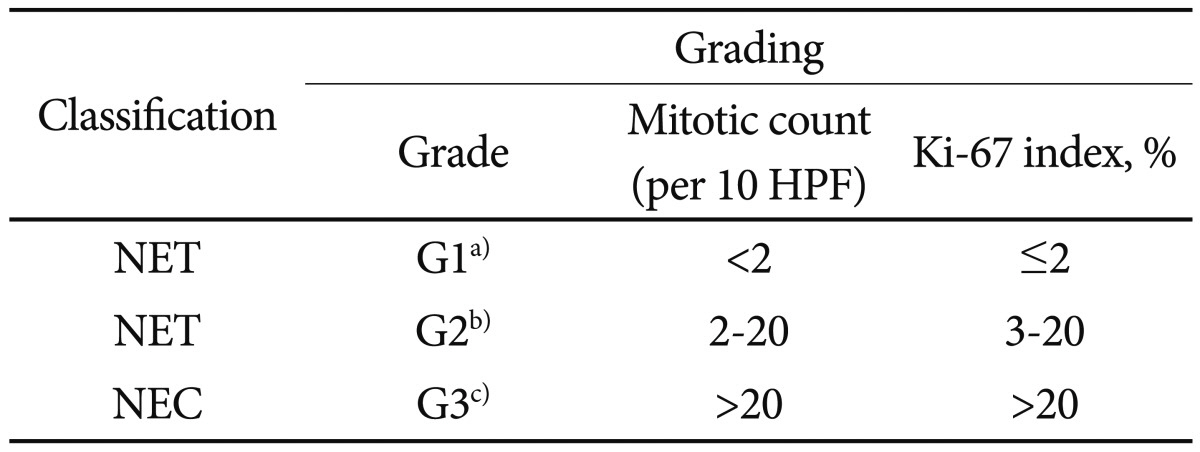
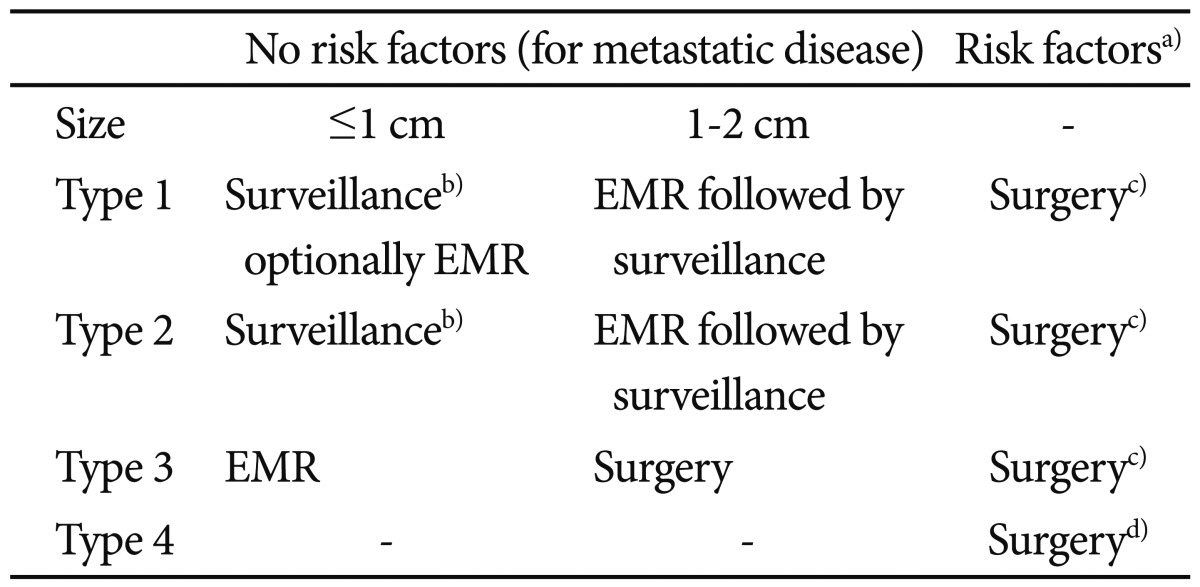
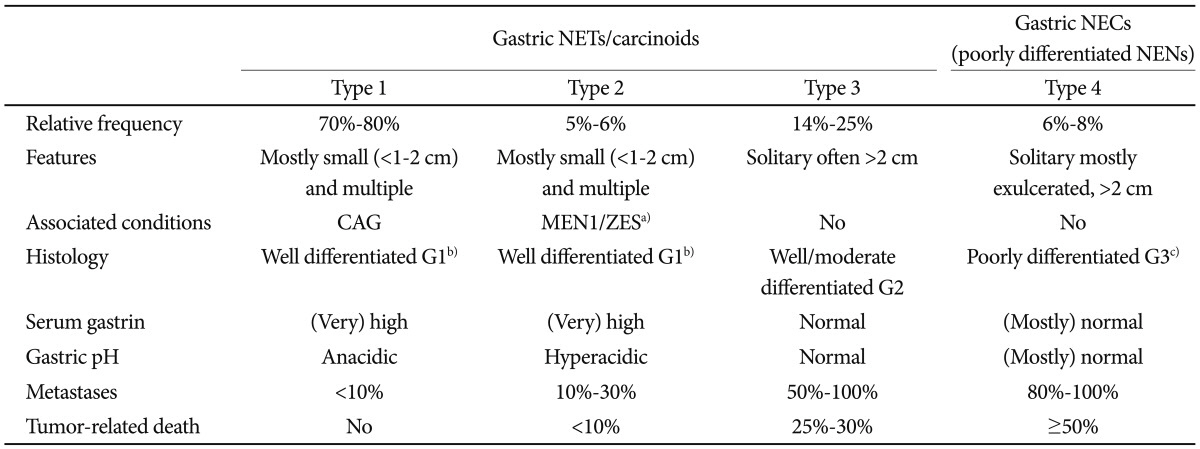




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download