Abstract
Rapid advances in the technology of gastrointestinal endoscopy as well as the evolution of science have made it necessary for us to continue update in either various endoscopic techniques or state of art lectures relevant to endoscopy. International Digestive Endoscopy Network (IDEN) 2013 was held in conjunction with Korea-Japan Joint Symposium on Gastrointestinal Endoscopy (KJSGE) during June 8 to 9, 2013 at Seoul, Korea. Two days of impressive scientific program dealt with a wide variety of basic concerns from upper gastrointestine (GI), lower GI, pancreaticobiliary endoscopy to advanced knowledge including endoscopic submucosal dissection forum. IDEN seems to be an excellent opportunity to exchange advanced information of the latest issues on endoscopy with experts from around the world. In this special issue of Clinical Endoscopy, we prepared state of art review articles from contributing authors and the current highlights will skillfully deal with very hot spots of each KJSGE, upper GI, lower GI, and pancreaticobiliary sessions by associated editors of Clinical Endoscopy.
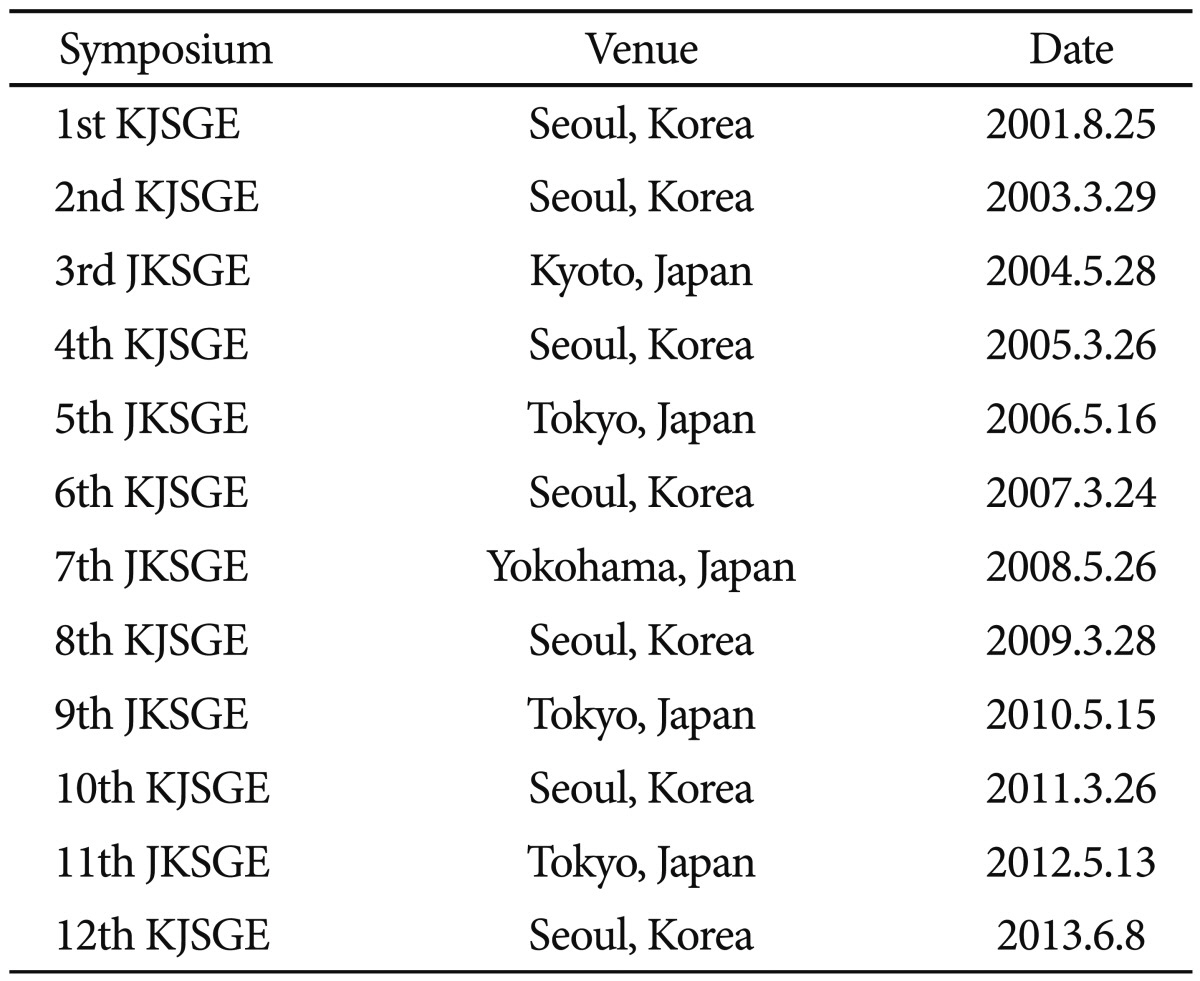
Joint symposium between the Korean Society of Gastrointestinal Endoscopy (KSGE) and Japan Gastrointestinal Endoscopy Society (JGES) has been held every year alternatively in Korea and Japan since 2001. 11th Japan-Korea Joint Symposium on Gastrointestinal Endoscopy (JKSGE) was held on May 13, 2012 during 83rd Congress of JGES in Tokyo, Japan. This year, 12th Korea-Japan Joint Symposium on Gastrointestinal Endoscopy (KJSGE) was held on June 8 in conjunction with International Digestive Endoscopy Network (IDEN) 2013 (Table 1). The IDEN 2013 organized by KSGE was held at Seoul, Korea on June 8 to 9, 2013. The invited lectures presented by renowned experts during KJSGE and IDEN 2013 are summarized to commemorate these courses with appropriate references.
The aim of joint symposium between KSGE and JGES is to promote mutual understanding and bilateral exchange of experience and knowledge in the field of endoscopy. Forty seven Japanese doctors including Michio Kaminish, the president of JGES, and eleven invited speakers participated in 12th KJSGE. There were active discussions between Japanese and Korean doctors during the meeting. Main topics of the joint symposium traditionally have been therapeutic endoscopy including bleeding control, endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD). Recently endoscopic retrograde cholangiopancreatography (ERCP), endoscopic ultrasonography (EUS), and EUS-guided fine needle aspiration (EUS-FNA) have been added to them.1 Two main topics of the 11th JKSGE were 'ESD for gastric cancer: current status and new developments' and 'therapeutic ERCP: current status and new developments.' The 12th KJSGE was composed of six sessions: long-term outcome of expended ESD for early gastric cancer (EGC), debates on colorectal ESD, two ESD/EMR forums, and two ERCP/EUS forums.
EGC is defined as a cancer confined to the mucosa or submucosa. Decision making to do ESD or surgery is based on the risk of lymph node metastasis. Traditionally, the criteria for endoscopic resection are limited by technical difficulty. Expanded criteria have been proposed2 because lymph node metastasis is almost free within the criteria and up to date techniques and devices make it possible to remove a larger lesion en bloc. Gotoda et al.3 from Tokyo Medical University presented their original data, which were the basis of the expanded criteria, and also suggested long-term favorable outcomes in the gastric cancer meeting the criteria. However, the expanded criteria were determined based on a retrospective examination of surgical cases for lymph node metastasis rates. A few long-term data are available by case series. Thus, Japanese gastric cancer treatment guideline suggested that the evidence regarding the curability of the ESD technique under the expanded criteria remains insufficient, and the ESD should be offered with caution for these indications.2 A prospective large study is now ongoing to confirm the expanded indication in Japan.4 A recent Korean retrospective study also showed comparable long-term outcome in terms of disease-free survival despite less favorable complete resection rate of expanded group compared those of guideline indication group,5 although we have to wait for the accumulation of more data in this patient group.
ESD is recommended for undifferentiated type EGC (UD-EGC) sized 2 cm or less without ulceration in the expanded criteria, but the clinical outcome data is limited.2 Short-term outcomes of complete resection and en bloc resection rates are less favorable to absolute (guideline) indication.5 Recently, Okada et al.6 suggested that ESD for UD-EGC may yield good long-term outcomes. In this session, Dr. Oda from National Cancer Center, Tokyo, presented their data of 113 UD-EGC. En bloc resection and R0 resection rates were 99.1% and 90.3%, respectively. However, curative resection rate meeting the expanded criteria for UD-EGC in post-ESD pathology specimen was poor showing only 63.9% (62/97). In 46 patients who was evaluated for long-term outcome after curative resection, 5 years overall survival was 93.0%. None of them had local or distant recurrence at all. ESD for UD-EGC can provide excellent 5-year survival rate in patients who achieved curative resection. Further surgery should be offered if the curative resection was not achieved.
There was a discussion whether there is a difference in the indication of colorectal ESD between Japan and Korea. Japanese Society for Cancer of Colon and Rectum published a guideline for the treatment of colorectal cancer (CRC) in 2010.7 Endoscopically treatable CRCs are mucosal or shallow submucosal invasive cancers less than 2 cm in size. Though there is no concrete indication for colorectal ESD in Korea, Korean endoscopists also apply the same indication in their practice. Close observation for the gross morphology of the tumor using narrow band image (NBI) with or without magnifying endoscopy is essential to select adequate cases for ESD.
There was a discussion whether the gravity alone is enough or other traction device is necessary for effective dissection. Techniques such as sinker-assisted ESD, traction with clip and nylon string, and cross-counter technique with a novel large-diameter balloon overtube were discussed. A comprehensive review about using the gravity for colorectal ESD will be presented in this issue of Clinical Endoscopy.
There were eleven presentations in this session. New full-thickness resection techniques with nonexposed endoscopic wall inversion surgery had drawn interests. This procedure is a combination of laparoscopic circumferential seromyotomy and seromuscular suturing with endoscopic circumferential mucosubmucosal incision. This technique enabled safe and effective en bloc full thickness resection without intra-abdominal contamination and looked quite promising. In addition, risks of synchronous and metachronous tumors during and after ESD were discussed.
There were also eleven presentations in this session. Many of the presentations were related with biliary stenting including antireflux metal stent and stent in stent technique. Interesting presentations were about ERCP performed with short double balloon endoscope. Usefulness and safety of short double balloon endoscope for diagnostic and therapeutic ERCP in patients with altered gastrointestinal anatomy were discussed. This procedure expected to become popular in the future.
ESD is increasingly gaining popularity in Eastern countries to achieve en bloc and complete resection of larger lesions, but the procedure is challenging because of long procedure time and higher risk of complications. Although new technology and devices are emerging, it is still a difficult procedure and requires a high level of endoscopists' skill. The most difficult portion to perform ESD is the fundus and corpus greater curvature side of the stomach. Endoscopic resection should be performed under moderate sedation combining drugs for analgesia and sedatives to provide comfort for patients and safer procedure for endoscopists. Perforation occurs in almost 4% during ESD and risk factors are location (upper part of the stomach), size, and ulcer finding. Perforation should be closed using endoclips and, along with adequate post procedure care (antibiotics and starvation), patients can usually recover completely without any morbidity.
During ESD, endoscopists sometimes encounter submucosal fibrosis under the lesion, which usually results from previous benign inflammation or tumor infiltration. If the submucosal fibrosis is severe, ESD procedure will take more time and complications, such as perforation and immediate bleeding, will occur more frequently.8 To perform resection for tumors with considerable fibrosis, dissection should be started far from the fibrotic portion to evaluate anatomic relation of proper muscle layer and fibrotic submucosal layer. Knife with a firm body such as a fixed Flex knife would be more use to dissect fibrotic portion.
Bleeding during and after procedure is the most common problems of ESD and occurs more frequently than in EMR. Endoscopic hemostasis should be performed immediately once bleeding occurs. Basic skill to prevent bleeding is injection and coagulation with electrosurgical knives. During procedure, it is more desirable to prevent bleeding by coagulating large submucosal vessels before cutting. Because blind dissection can cause severe bleeding by cutting large perforating vessels during procedure, direct visualization of submucosal layer is very important to prevent bleeding. Several traction techniques will be helpful for better visualization of the dissection field.
ESD for EGC in remnant stomach after subtotal gastrectomy or in gastric tube after esophagectomy is a technically difficult procedure because of the limited working space of endoscope and presence of severe fibrosis or staples at the suture line.9 Dr. Oda presented their recently published long-term outcome of ESD for EGC at remnant stomach. ESD was a treatment method of choice considering that it is less invasive than additional surgery, and the prognosis is excellent showing 87.3% and 100% of 5-year overall and cause specific survival rates, respectively.10 Although a high en bloc resection rate was achieved by ESD for EGC in a remnant stomach or gastric tube, this procedure is a challenging procedure due to the high complication rate of perforation for lesions in the expanded indication.11
Upper gastrointestinal bleeding (UGIB) is the most common emergency in the field of gastroenterology and endoscopy has a clearly-defined role in the primary management of UGIB.12 The endoscopic hemostatic therapy for nonvariceal UGIB (NVUGIB) is indicated for the patients having ulcers with high risk bleeding stigmata such as active bleeding or nonbleeding visible vessels. Endoscopist can choose hemostatic method according to the type, size, ulcer base characteristics, and location of the lesion. Prof. Il Kwun Chung from Soonchunhyang University College of Medicine reviewed various endoscopic tools and materials which are suitable for application for UGIB. Injection therapy using epinephrine, ethanol, or hypertonic saline, thermal coagulation using contact and noncontact devices has been common choices in NVUGIB with good outcomes. Endoscopic mechanical modalities currently available can provide secure bleeding control in most cases. Various forms of hemoclips are now available, and combination of detachable snaring and clipping also can be used. Endoscopic hemostasis using a combination of currently available methods is preferred to monotherapy, especially to injection therapy alone, considering the theoretical additive or synergistic effect of each modality having different mechanism of action.
This topic was summarized and presented by Prof. James Y.W. Lau from the Chinese University of Hong Kong. Over-the scope-clip can overcome the limitation of usual hemoclips by allowing compression of larger amount of tissue, resulting in more secure hemostasis with high success rate even after failure of conventional hemostatic techniques.13 A newly developed inverted overtube allows endoscopists to perform the hemostatic procedures from the conventional position (left side of the patient) without changing the location of endoscopy units while freely and easily changing the patient's position to right decubitus.14 This technique improves the endoscopic views and subsequently shortens procedure time by moving blood clots and food residue away from the fundus or upper body. A new polysaccharide hemostatic system (EndoClot Plus Inc., Santa Clara, CA, USA) was recently developed for bleeding control in gastrointestinal tract endoscopy. Spray of this agent can effectively achieve hemostasis for controlling and preventing EMR-related bleeding with the advantage of simple application.15
Although endoscopy is the first diagnostic and therapeutic option in UGIB, transcatheter arterial embolization (TAE) is frequently used because of improvement in interventional devices, embolic materials, and wider availability of skillful intervention radiologists.16 Prof. Ji Hoon Shin from Asan Medical Center suggested that TAE is now the accepted salvage treatment that should be considered before surgery in massive refractory or recurrent UGIB.17 This technique has no absolute contraindication, but renal insufficiency, allergy, and uncorrectable coagulopathy are relative contraindications. In case of massive bleeding, surgery may be preferable to TAE. The only direct sign of UGIB is extravasation of contrast medium into the bowel lumen. If there's no active bleeding, indirect signs suggest recent bleeding focus, but correlation of angiographic finding with endoscopic finding. Commonly used embolic agents are metallic coil. Because the use of coil alone is frequently associated with rebleeding, polyvinyl alcohol particles or gelatin sponge are used in combination with coil. According to the Prof. Ji Hoon Shin's summary, TAE for NVUGIB showed 92% to 100% of technical success rate, and 51% to 94% of clinical success rates. Even if there's no active bleeding during angiography, prophylactic TAE can be done under the guidance of endoscopic information. TAE in the upper gastrointestinal (GI) tract above the Treitz ligament is safe because of rich collateral vessels around the duodenum and stomach. Safety and efficacy of TAE for NVUGIB for refractory bleeding to endoscopic treatment is widely accepted even in high operative risk patients.
Achalasia is an idiopathic motility disorder with impaired lower esophageal sphincter relaxation and loss of esophageal peristalsis. Peroral endoscopic myotomy (POEM) has been introduced as a novel endoscopic procedure to treat achalasia.18 Prof. Su Jin Hong from Soonchunhyang University presented 24 successful cases of POEM and showed that the technique is a feasible, safe, less invasive, and effective treatment, which can replace the established treatment in the future. Current endoscopy system provide on demand air insufflations system which may cause over distension of the gut lumen and may cause patients discomfort. Prof. Joo Young Cho from Soonchunhyang University presented a newly developed overtube system for steady pressure automatically controlled endoscopy.
Esophageal adenocarcinoma is a challenging problem to physicians and more than 95% of them are presented as a symptomatic advanced disease. According to a recent report, Barrett esophagus is a strong risk factor for esophageal adenocarcinoma, but the absolute annual risk, 0.12%, is much lower than the assumed risk of 0.5%, which is the basis for current surveillance guidelines.19 This finding raises the question about the rationale for ongoing surveillance in patients who have Barrett esophagus without dysplasia. Thus, defining at risk population is important and increased risk of Barrett esophagus is noted along with the history of chronic reflux disease, Caucasian males, visceral obesity, the use of tobacco, and family history of Barrett esophagus or adenocarcinoma. The use of standard endoscopy screening does not appear to be cost effective; office based transnasal endoscopy may be cost effective but requires a skillful endoscopist. A novel cytosponge was suggested to be cost effective in a Markov model in screening 50-year-old men with gastroesophageal reflux disease.20 More technically advanced method of volume laser endomicroscopy has been introduced but is still in the early stage of clinical translation.
Endoscopic forceps biopsy is essential before planning an endoscopic resection of upper GI epithelial tumors. Because forceps biopsy is limited by its superficiality, discrepancy between the initial endoscopic biopsy and resected specimens are not rare. Suggested factors associated with such discrepancies are large tumor size, depressed morphology, and surface erythema.21 Multiple biopsies increase the diagnostic yield but can induce mucosal ulceration, which may cause the difficulty of procedure and subsequent complications. Exact targeting is more complicated than the collection of multiple biopsy specimens. Rebiopsy at the referral hospital may have several disadvantages such as patient discomfort, biopsy-induced ulcer, and fibrosis. However, repeated biopsy might be recommended if the result of previous one is confusing. The recommended interval between biopsy and ESD is less than 3 weeks because of the fibrosis and nonlifting sign in CRC, but it is uncertain whether this can be also applied to ESD for gastric lesions.22
Evaluation of subepithelial lesions with EUS allows further characterization of the mass, but imaging alone is not sufficient in establishing diagnosis. EUS-FNA has been suggested to be a reliable method for obtaining tissue, and FNA needles ranging 19 to 25 gauges yield cellular material that can be evaluated by cytologist. For definite diagnosis, sufficient cellular material is needed to obtain a cell block and to perform immunohistochemistry with cKIT, DOG-1, SMA, and S100. A recent report says EUS-FNA sampling of subepithelial lesions was diagnostic in 61.6% and showed a spindle cell neoplasm (suspicious) in another 22.3% (diagnostic yield 83.9%).23 To augment diagnostic yield, core biopsy was suggest for sufficient tissue acquisition. The first widely used core biopsy needle was the Trucut needle (Quickcore; Cook Medical, Winston-Salem, NC, USA). This method increases diagnostic yield in cases with failed EUS-FNA, but not higher than FNA if used alone.24
Upper GI obstruction due to esophageal, gastric, and duodenal malignancies, including periampullary cancer, is not an uncommon situation in far advanced cancer patients. The success rates of stents are around 95% for technical success and more than 80% to 90% for clinical success. Technical success rate depends on the site and characteristics of the lesion, including anatomic location, severity of stenosis, and acute angulation. Upper GI stents can be placed under fluoroscopic guidance, endoscopic guidance or both. Fluoroscopy helps identification of stricture morphology and length and deformed anatomy. Thus, fluoroscopy guides the stent selection and reduces the risk of severe complication including perforation. Endoscopic guidance can reduce the procedure duration by making the guide wire passage through stenotic portion somewhat easier.
Esophageal stents are commonly used for the palliation of malignant dysphagia obstruction. The indications for stent placement gradually expanded to encompass benign esophageal diseases, fistulae, and complex strictures. Because stents for benign diseases are usually temporary, safety in stent removal is an important feature. Uncovered or partially covered stents are difficult to remove because of tissue reaction (granulation tissue) and embedding of the stent after a prolonged placement. Covered stent in benign condition frequently migrates than in malignant obstruction, which also makes the removal difficult. Stent removal is recommended usually within 6 weeks. A recent large retrospective study showed that esophageal stent removal in the benign disease was a safe and feasible procedure having 2.1% of major adverse event rate, and fully covered self-expandable metal stents (SEMSs) were more successfully removed than other types of stents.25 Biodegradable stents can overcome some problems of SEMSs and the main advantage seems to be the fact that endoscopic removal is not needed. Covered biodegradable stents will be available in the future to treat benign esophageal perforations/leaks as well as for treating same malignant indications.26
Wireless capsule endoscopy (WCE) is currently approved by U.S. Food and Drug Administration for use in adult and pediatric patients aged 10 years or older for evaluation of occult gastrointestinal bleeding, Crohn disease, celiac disease, polyposis syndromes, small bowel abnormalities detected on imaging studies, and symptom evaluation. New investigations have focused on optical improvements, advances in intestinal cleaning and risk reduction strategies to optimize WCE methodologies in clinical practice and to expand the potential utility of WCE, novel devices that can maneuver within or insufflate the gut lumen, tag or biopsy suspect lesions, or target drug delivery to specific sites are in development.
Double-balloon enteroscopy (DBE) enables endoscopic treatment of small intestinal diseases that historically required open surgery. For example, endoscopic hemostasis, accurate diagnosis of the bleeding source, endoscopic polypectomy, and endoscopic dilation of small intestinal strictures may avoid surgical exploration and possible resection of the intestine. It is important to maintain good control of endoscope by arranging the shape of the endoscope shaft for effective procedures of balloon assisted enteroscopy. Transparent hood, CO2 insufflation and submucosal injection are useful tips to make endoscopic treatment in the small bowel easier and safer.
Three deep enteroscopy methods are currently available: DBE, single-balloon enteroscopy (SBE), and spiral enteroscopy (SE). Although the clinical impact of total enteroscopy rates remains controversial, the results of previous studies suggest that DBE, SBE, and SE have comparable diagnostic and therapeutic yields. Therefore, the selection of an enteroscopic technique should be based on availability and the endoscopist's experience.
The selection of therapy for early Barrett's related esophageal adenocarcinoma depends heavily on three major factors. The first is expertise of the performing physician. The second is the commitment of the patient. The other critical factor is the lesion itself. Once these factors can be ensured, endoscopic resection with ablation can be carried out.
Foregut neuroendocrine tumors (NETs) seem to have a broad range of clinical behavior from benign to metastatic. The treatment of choice for a localized NET is usually surgery. Many foregut NETs are diagnosed at an early stage because of the advent of screening endoscopy, and thus can be managed with endoscopic treatment because of a low frequency of lymph node and distant metastasis. Endoscopic treatment may be one of therapeutic options for eligible foregut NETs. However, the appropriate selection criteria of foregut NETs for endoscopic resection is still controversial and further studies are needed.
In rectal NETs of <10 mm in size, endoscopic treatment might be most feasible and both EMR/EMR-C and ESD showed similar efficacy and safety. However, although endoscopic treatment could be safely performed on highly selected cases when the tumor size is 11 to 15 mm, surgery is still recommended for rectal NETs larger than 10 mm because of the higher risk of the need for additional rescue therapy and metastasis later.
Prof. Bong Min Ko gave a lecture on dye-based image-enhanced endoscopy which include indigocarmine and crystal violet. In some studies, panchromoendoscopy improves detection of adenoma in the colon. Also, chromoscopy in inflammatory bowel disease is beneficial for improving detection rate of colitis-associated neoplasm. Chromoscopy is useful for distinguishing adenoma from hyperplastic polyps.
NBI technique is developed for finding and observing subtle mucosal abnormality and, especially in colon, this is useful for detecting or differentiating epithelial tumors (polyps). Despite clearly defined benefits in clinical application of this technique, there are unsettled areas, such as screening and surveillance of colon cancer or pathology assessment.
Prof. Yasushi Sano gave a lecture on the efficacy of magnifying chromoendosocopy and magnifying colonoscopy with NBI for detection, histological prediction, estimation of the depth of early CRC, and future prospects.
Prof. Joo Ha Hwang gave a lecture on the use of probe based confocal microscopy (pCLE), which allows real-time in vivo microscopy to evaluate the microarchitecture of the mucosal epithelium. It appears to be particularly useful in identifying mucosal dysplasia and early malignancies that cannot be distinguished using high-definition white light endoscopy, chromoendoscopy, or magnification endoscopy.
In 2012, the United States Multi-Society Task Force on CRC issued a guideline for colonoscopy surveillance after screening and polypectomy,27 which updated the previous 1997 and 2006 guidelines. The British Society of Gastroenterology updated their 2002 surveillance guideline in 2010.28 They recommend an interval of 10 years after negative findings on baseline colonoscopy and an interval of 5 to 10 years after detection of low risk adenomas for average-risk individuals, assuming that the baseline colonoscopy is complete with a good bowel preparation. The Korean guideline published in 2012 also recommended an interval of 5 years after index colonoscopy for people without high-risk findings.29 However, there is still a large gap between a real practice and a guideline.
The imaging equipment used for endoscopy has evolved enough to replace the pathologic report. NBI with magnification colonoscopy is useful for histological prediction and for estimating the depth of invasion of CRC. To standardize the currently available diagnostic strategies, the NBI international colorectal endoscopic classification would be helpful for endoscopists irrespective of whether they have access to a magnifying endoscopy. However, a more prospective research is needed in order to prove that this international classification can be applied with satisfactory availability, feasibility, and reliability. In the near future, NBI might make a valuable contribution to real-time histological prediction during colonoscopy, which would have substantial benefits for reducing both the risk of polypectomy and the costs of histological evaluation by allowing adenomatous polyps to be resected and discarded.30
Several quality parameters have become apparent. These include quality of the bowel preparation, cecal intubation, adenoma detection rate, withdrawal time, polyp retrieval rate, burden of colonoscopy, and complications during colonoscopy. The quality of bowel preparation can be enhanced by split-dose regimens, which are superior to single-dose regimens. Cecal intubation rates should be approximately 95% and can be optimized by use of good techniques. In selected patients, specific devices can be used to facilitate cecal intubation. Adenoma detection rates should be monitored and should exceed a minimum of 25% in men and 15% in women. To this aim, optimal withdrawal technique and adequate time for inspection are of utmost importance. Of all advanced imaging techniques, chromoendoscopy is the only technique with proven benefit for adenoma detection. Finally, the technique of polypectomy affects the number of complications as well as the success of complete removal of a lesion.31
There is a lack of specific studies based on the setting of screening. The current indications for colon capsule are: if colonoscopy is impossible for technical reasons, if there are contraindications to colonoscopy or if the patient is reluctant to undergo high-definition colonoscopy. The choice of a screening test includes several factors, such as cost, invasiveness, acceptability, adherence to repeat tests, and acceptance of a referral for colonoscopy for positive tests as well as local financial resources. Video colon capsule is a new tool for exploration of the colon; however, further studies are required before it can become a screening test.32
Because most lower gastrointestinal bleeding (LGIB) is self-limiting, colonoscopy has been performed after the bleeding had stopped and when the patient was adequately prepared. However, urgent colonoscopy was performed for continuous bleeding. Signs of hemodynamic instability were pallor, fatigue, palpitations, chest pain, dyspnea, tachypnea, and tachycardia. In particular, a decrease in standing systolic blood pressure more than 10 mm Hg or an increase in heart rate more than 10 times per minute, indicated more than 15% effective blood loss. In patients with chronic LGIB, the principle of treatment is based on selective performance of colonoscopy after appropriate colonic preparation. However, early colonoscopy could be performed in patients with acute LGIB after the correction of hemodynamic instability. It is unclear whether unprepared colonoscopy is more effective as compared to prepared colonoscopy with few randomized controlled trials on this subject, but most endoscopists seem to prefer prepared colonoscopy.33,34
Surgery has been the mainstay of management of colonic perforation. Endoscopic clipping has recently been introduced and conservative management has been feasible in many cases of perforation. However, surgery is still indicated in cases of large perforations, generalized peritonitis, aggravating peritonitis, ongoing sepsis, and concomitant colorectal pathology, such as large advanced neoplasm, which is difficult to resect using endoscopic techniques.35 Through the scope clips have been used in clinical practice for decades with a satisfactory success rate for management of colonoscopy-related perforation.36 They are especially useful in the closure of small perforations, such as those developing after EMR or ESD of colorectal tumors. Large perforations may not be closed using through the scope clips only. In those cases, combination of clips and a detachable snare (endoloop) can be useful in endoscopic closure. In a recent study, over the scope clips were reported to be useful for the management of large gastrointestinal perforations.37
Considerable debate surrounds the choice of the most appropriate option. Initially, staged procedures were advocated in order to decrease morbidity and mortality. Currently, it appears that single-stage procedures provide similar or even better outcomes. Endoluminal stenting has been shown or described to provide effective relief of obstruction with few procedural complications. Compared to the surgical interventions, endoluminal stenting is a relatively new technique and has generated new discussions regarding the most appropriate treatment for malignant large bowel obstruction. Prof. Soren Meisner presented, on the basis of systematic reviews cited in his lecture, that randomized controlled trials should be conducted with a focus on survival, quality of life, and cost effectiveness, in high volume centers, and focusing on oncological outcome and selection criteria for best treatments, and that stenting provided an increased number of primary anastomoses, reduced stoma creation, and reduced overall complications.38-41
Prof. Seun Ja Park lectured on an overview of useful accessories for colorectal EMR, such as cap and band. She emphasized that the cap-assisted colonoscopy was useful in a diagnostic colonoscopy as well as in therapeutic endoscopies.42
Prof. Shinji Tanaka introduced a variety of newly developed electrosurgical knives for colorectal ESD. He demonstrated the basic strategy in selecting a knife based on the characteristics of each knife and the situation of the lesion in colorectal ESD.
The sinker allowed direct visualization of the cutting line, and en bloc resection was performed successfully. Other traction systems that facilitate colorectal ESD procedures have been reported, such as the small caliber tip transparent hood (ST hood), the magnetic anchor system, sheath-assisted counter traction, thin endoscope-assisted ESD, the medical ring and the clip-band technique.43
Prof. Soren Meisner presented a video lecture based on several difficult emergency cases with clinical total obstruction, and introduced the public website (www.weshareendoscopy.com) for endoscopists. The use of marking with lipiodol injection in the most difficult locations for stenting, such as just oral to splenic flexure, was explained. He demonstrated the use of the ERCP balloon extractor catheter for injection of luminal contrast and making very precise tumor delineation in the tumor, which was fixed transversely and was very difficult to pass and delineate. The papillotome was used in very difficult position of endoscope-passage of the guidewire and catheter. Placement of a guidewire in placement of stent must be through the lumen and not through any mesh holes.
The chance of developing reobstruction after successful stenting in patients with CRCs has increased. Although, theoretically, covered stents have been developed for reduction of stent reobstruction by blocking tumor infiltration and increasing the stent patency, recent studies failed to show any clinical advantage of covered stents with high incidence of stent migration. Primary colectomy after successful endoscopic stenting could be an alternative therapeutic option in unresectable CRCs, especially in patients with expected long-term survival. Second stent is an alternative treatment for the relief of malignant colorectal obstruction, and palliative surgery should be considered for patients who show good performance and in whom long-term survival is expected.
Prof. Hironori Yamamoto lectured on practical application and secrets in endoscopic dilation and stenting using DBE. DBE enabled endoscopic treatments such as balloon dilation and stent placement for small intestinal strictures. Endoscopic evaluation of a stricture before treatment is important in order to rule out malignant strictures. A transparent hood, especially an ST hood, is useful in dilation therapy, and CO2 insufflation is useful in therapeutic DBE such as balloon dilation. Dilation size in the small intestine is usually limited to up to 12 to 15 mm.
Benign colorectal strictures can develop after diverticulitis, ischemic colitis, radiation colitis, colorectal ESD, or colonic resection. Most strictures could be managed successfully with several treatment modalities, such as direct digital dilation, transanal surgical treatment, endoscopic bougies, endoscopic balloon dilation, and stent insertion. However, other varieties of endoscopic or surgical techniques are required in refractory strictures, especially after the failure of first endoscopic management. Biodegradable stents have been introduced, which can maintain an adequate lumen across anastomotic strictures that are resistant to balloon dilation.
The pancreatobiliary region has one of the most complicated structures and physiological functions in our body. Endoscopic procedure in the region thus requires rather complicated techniques. In IDEN 2013, many varied cases associated with basic procedures to advanced techniques of ERCP were introduced and great enthusiastic lectures were given by invited experts. Also "direct visualization of bile duct and pancreatic duct" and "tips for successful ERCP in surgically altered anatomy" were presented in video forum. Here, I will give a summarized review about extracorporeal shock wave lithotripsy (ESWL) and endotherapy for pancreatic duct stones, endoscopic management of ERCP-related complications, technical advances in EUS-guided tissue acquisition for pancreatic lesions, challenging issues on EUS-assisted transmural drainage, and current topics on biliary stenting for hilar cholangiocarcinoma.
Pancreatic duct stones are found in approximately 22% to 60% of patients with chronic pancreatitis (CP). These stones can lead to the obstruction of the outflow of pancreatic juice, causing increased intraductal pressure, tissue hypertension and ischemia, which may be major factors causing pain in patients with CP. Guda et al.44 conducted a meta-analysis including a total of 588 patients and found that ESWL was effective in relieving main pancreatic duct obstruction and in alleviating pain in CP, most often in combination with endoscopic therapy. Stone fragmentation is considered successful when one of the following findings is seen on plain films following ESWL: 1) decreased stone density, 2) increased stone surface, and 3) heterogeneity of the stone, which appears as powder-like material filling the pancreatic and the surrounding secondary ducts.
Hemorrhage as a complication of ERCP may occur during or up to 10 days after the procedure. It is most often seen in the setting of a sphincterotomy, and it takes place in 1.3% of the time. Itoi et al.45 had several strategies for hemostasis for the postendoscopic biliary sphincterotomy (post-EBS) bleeding or acute bleeding during stent exchange. Although this study had the limitations of a small sample size and lack of control patients, covered SEMS placement for endoscopic hemostasis may be useful in selected patients with uncontrolled post-EBS bleeding. Gut perforations during ERCP with sphincterotomy or due to migration of stents are very rare complications, with an incidence of well below 2%. Moreover, direct duodenoscope-induced lateral or medial duodenal wall perforation is much less common, accounting for 0.1% of patients who undergo ERCP, but tends to be large and further away from the ampulla. The standard treatment for traumatic or iatrogenic duodenal perforation is early surgical closure because of a relatively high mortality rate of 16% to 18%. Recently, numerous endoscopic trials of perforation management have increased and successful primary repair of duodenal perforation using the endoscope itself has been reported.
There was a notable lecture about advanced techniques for pancreaticobiliary visualization in video lecture in IDEN 2013. Spyglass direct visualization system has been recently developed for single-operator examination of the bile duct and pancreatic duct. It consists of three components; Spyscope, optic probe, and Spybite biopsy forceps. It can be used for both diagnostic and therapeutic purposes. Although Spyglass system has some limitations, it is safe, easy to perform, and will be more popular as technology advances. Peroral cholangioscopy (POC) permits direct visualization of the biliary tree for diagnostic procedures and provides endoscopic guidance for therapeutic interventions. An ultraslim upper endoscope developed for transnasal upper endoscopy could be advanced into the bile duct through the major papilla. Direct POC using an ultraslim endoscope has been proposed as a single-operator system for direct endoscopic examination of the biliary tree. Direct visualization of biliary system has advantages in identifying and treating intraductal lesions compared to indirect imaging of ERCP. Percutaneous transhepatic cholangioscopy has been the most widely used modality for diagnosis and treatment for biliary diseases. Peroral pancreatoscopy have been reported to be useful in intraductal papillary mucinous neoplasia (IPMN) for differential diagnosis between malignant IPMNs and benign ones, and classification of histopathological subtypes.
EUS-FNA has been increasingly used for diagnostic and staging purposes of solid pancreatic lesions or ductal carcinoma. EUS-FNA has been shown to detect tumors less 3 mm, due to high spatial resolution allowing the detection of very small lesions and vascular invasion, particularly those in the pancreatic head and neck, which may not be detected on transverse computed tomography. Furthermore, this minimally invasive procedure is often ideal in the endoscopic procurement of tissue in patients with unresectable tumors. The concept of tailoring chemotherapy based on molecular markers has increased the demand for core tissue by means of EUS-guided fine needle biopsy. Varadarajulu et al.46 found that tissue acquisition, including transduodenal passes, was successful and adequate for cytologic assessment in all 38 patients (100%). Satisfactory histologic specimens were procured from 36 of 38 patients (94.7%). An onsite diagnosis was established in 35 of 38 patients (92.1%). The currently available ProCore and Flexible 19 gauge needles are a significant advancement to acquiring core tissue during EUS-guided procedures.
Currently, ERCP is the preferred procedure for biliary drainage (BD) for various pancreaticobiliary disorders. Indications for EUS-BD include failed conventional ERCP, altered anatomy, tumor preventing the access into the biliary tree and contraindication to percutaneous access. Recently, EUS-guided pancreaticogastrostomy (EUS-PG) or pancreaticoduodenostomy (EUS-PD) have been reported as an alternative method for reducing ductal hypertension in patients with CP. Jang et al.47 analyzed 22 symptomatic patients with pancreatic duct obstruction who are unsuitable or unsuccessful for transpapillary drainage. EUS-PG or EUS-PD were successful in eighteen patients (82% [18/22], technical success rate). Fourteen patients showed decrease in pain score more than 3 point within 2 months (64% [14/22] as intention treat, and 82% [14/17] as protocol; clinical success rate). In his case series, EUS-PG or EUS-PD may be feasible and safe, and can be used as an alternative option to surgery.
Endoscopic biliary stenting has been performed since 1979. Two different materials, plastic or metallic, are available for the stenting. The plastic stent is used for benign stenosis. When a patient is inoperable, we usually perform the endoscopic stenting using uncovered SEMS for Klatskin tumor. When a patient's condition was well, chemotherapy and radiotherapy are added. Endoscopic stenting, chemotherapy and radiotherapy improved the patient's quality of life. Uncovered metal stent is longer than plastic stent in the hilar malignant stenosis. Although endoscopic biliary metal stenting is the mainstay of palliative treatment in patients with unresectable hilar cholagiocarcinoma,48 tumor ingrowth or overgrowth is a significant problem of uncovered stents. Several clinical trials have reported the therapeutic effect of photodynamic therapy (PDT) for unresectable hilar cholangiocarcinoma. The ability of PDT to destroy cancer and neovascular cells may prolong stent patency. However, the effect of PDT on stent patency has yet to be determined.
ERCP in patients with surgically altered anatomy is always challenging. Recently, DBE and SBE have been used not only for diagnostic and therapeutic endoscopy of the small bowel but also for ERCP-related procedures. Various techniques have been also introduced for the successful ERCP in patients with Billroth-II gastrectomy, including using a cap-fitted forward-viewing endoscope. A thorough understanding of a surgically altered anatomy is essential to minimize complications and to bring about successful treatment.
References
1. Kaminishi M, Niwa H. Past, present, and future of the Korea-Japan Joint Symposium on Gastrointestinal Endoscopy. Clin Endosc. 2011; 44:1–5. PMID: 22741105.

2. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011; 14:113–123. PMID: 21573742.
3. Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000; 3:219–225. PMID: 11984739.

4. Oda I, Shimazu T, Ono H, et al. Design of Japanese multicenter prospective cohort study of endoscopic resection for early gastric cancer using Web registry (J-WEB/EGC). Gastric Cancer. 2012; 15:451–454. PMID: 22549754.

5. Park CH, Shin S, Park JC, et al. Long-term outcome of early gastric cancer after endoscopic submucosal dissection: expanded indication is comparable to absolute indication. Dig Liver Dis. 2013; 45:651–656. PMID: 23422031.

6. Okada K, Fujisaki J, Yoshida T, et al. Long-term outcomes of endoscopic submucosal dissection for undifferentiated-type early gastric cancer. Endoscopy. 2012; 44:122–127. PMID: 22271022.

7. Watanabe T, Itabashi M, Shimada Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol. 2012; 17:1–29. PMID: 22002491.

8. Jeong JY, Oh YH, Yu YH, et al. Does submucosal fibrosis affect the results of endoscopic submucosal dissection of early gastric tumors? Gastrointest Endosc. 2012; 76:59–66. PMID: 22726467.

9. Lee JY, Choi IJ, Cho SJ, et al. Endoscopic submucosal dissection for metachronous tumor in the remnant stomach after distal gastrectomy. Surg Endosc. 2010; 24:1360–1366. PMID: 19997930.

10. Nonaka S, Oda I, Makazu M, et al. Endoscopic submucosal dissection for early gastric cancer in the remnant stomach after gastrectomy. Gastrointest Endosc. 2013; 78:63–72. PMID: 23566640.

11. Nishide N, Ono H, Kakushima N, et al. Clinical outcomes of endoscopic submucosal dissection for early gastric cancer in remnant stomach or gastric tube. Endoscopy. 2012; 44:577–583. PMID: 22402983.

12. Hwang JH, Fisher DA, Ben-Menachem T, et al. The role of endoscopy in the management of acute non-variceal upper GI bleeding. Gastrointest Endosc. 2012; 75:1132–1138. PMID: 22624808.

13. Manta R, Galloro G, Mangiavillano B, et al. Over-the-scope clip (OTSC) represents an effective endoscopic treatment for acute GI bleeding after failure of conventional techniques. Surg Endosc. Epub 2013 Feb 23. DOI: 10.1007/s00464-013-2871-1.

14. Mori H, Kobara H, Fujihara S, et al. Accurate hemostasis with a new endoscopic overtube for emergency endoscopy. World J Gastroenterol. 2013; 19:2723–2726. PMID: 23674883.

15. Huang R, Pan Y, Hui N, et al. Polysaccharide hemostatic system for hemostasis management in colorectal endoscopic mucosal resection. Dig Endosc. Epub 2013 Mar 31. DOI: 10.1111/den.12054.

16. Loffroy R, Estivalet L, Cherblanc V, et al. Transcatheter embolization as the new reference standard for endoscopically unmanageable upper gastrointestinal bleeding. World J Gastrointest Surg. 2012; 4:223–227. PMID: 23467300.

17. Shin JH. Recent update of embolization of upper gastrointestinal tract bleeding. Korean J Radiol. 2012; 13(Suppl 1):S31–S39. PMID: 22563285.

18. Lee BH, Shim KY, Hong SJ, et al. Peroral endoscopic myotomy for treatment of achalasia: initial results of a korean study. Clin Endosc. 2013; 46:161–167. PMID: 23614126.

19. Hvid-Jensen F, Pedersen L, Drewes AM, Sørensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med. 2011; 365:1375–1383. PMID: 21995385.

20. Benaglia T, Sharples LD, Fitzgerald RC, Lyratzopoulos G. Health benefits and cost effectiveness of endoscopic and nonendoscopic cytosponge screening for Barrett's esophagus. Gastroenterology. 2013; 144:62–73.e66. PMID: 23041329.

21. Cho SJ, Choi IJ, Kim CG, et al. Risk of high-grade dysplasia or carcinoma in gastric biopsy-proven low-grade dysplasia: an analysis using the Vienna classification. Endoscopy. 2011; 43:465–471. PMID: 21425043.

22. Han KS, Sohn DK, Choi DH, et al. Prolongation of the period between biopsy and EMR can influence the nonlifting sign in endoscopically resectable colorectal cancers. Gastrointest Endosc. 2008; 67:97–102. PMID: 18155430.

23. Hoda KM, Rodriguez SA, Faigel DO. EUS-guided sampling of suspected GI stromal tumors. Gastrointest Endosc. 2009; 69:1218–1223. PMID: 19394006.

24. Storch I, Jorda M, Thurer R, et al. Advantage of EUS Trucut biopsy combined with fine-needle aspiration without immediate on-site cytopathologic examination. Gastrointest Endosc. 2006; 64:505–511. PMID: 16996340.

25. van Halsema EE, Wong Kee Song LM, Baron TH, et al. Safety of endoscopic removal of self-expandable stents after treatment of benign esophageal diseases. Gastrointest Endosc. 2013; 77:18–28. PMID: 23261092.

26. van Boeckel PG, Vleggaar FP, Siersema PD. Biodegradable stent placement in the esophagus. Expert Rev Med Devices. 2013; 10:37–43. PMID: 23278222.

27. Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012; 143:844–857. PMID: 22763141.

28. Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut. 2010; 59:666–689. PMID: 20427401.

29. Yang DH, Hong SN, Kim YH, et al. Korean guidelines for postpolypectomy colonoscopy surveillance. Clin Endosc. 2012; 45:44–61. PMID: 22741132.

30. Iwatate M, Ikumoto T, Hattori S, Sano W, Sano Y, Fujimori T. NBI and NBI combined with magnifying colonoscopy. Diagn Ther Endosc. 2012; 2012:173269. PMID: 23304065.

31. Hazewinkel Y, Dekker E. Colonoscopy: basic principles and novel techniques. Nat Rev Gastroenterol Hepatol. 2011; 8:554–564. PMID: 21894202.

32. Van Gossum A, Devière J. Colon capsule endoscopy: a new tool for colon examination? Discov Med. 2010; 9:46–50. PMID: 20102685.
33. Strate LL, Syngal S. Timing of colonoscopy: impact on length of hospital stay in patients with acute lower intestinal bleeding. Am J Gastroenterol. 2003; 98:317–322. PMID: 12591048.

34. Jensen DM. Current management of severe lower gastrointestinal bleeding. Gastrointest Endosc. 1995; 41:171–173. PMID: 7721011.

35. Raju GS, Saito Y, Matsuda T, Kaltenbach T, Soetikno R. Endoscopic management of colonoscopic perforations (with videos). Gastrointest Endosc. 2011; 74:1380–1388. PMID: 22136781.

36. Yang DH, Byeon JS, Lee KH, et al. Is endoscopic closure with clips effective for both diagnostic and therapeutic colonoscopy-associated bowel perforation? Surg Endosc. 2010; 24:1177–1185. PMID: 19915907.

37. Parodi A, Repici A, Pedroni A, Blanchi S, Conio M. Endoscopic management of GI perforations with a new over-the-scope clip device (with videos). Gastrointest Endosc. 2010; 72:881–886. PMID: 20646699.

38. Cirocchi R, Farinella E, Trastulli S, et al. Safety and efficacy of endoscopic colonic stenting as a bridge to surgery in the management of intestinal obstruction due to left colon and rectal cancer: a systematic review and meta-analysis. Surg Oncol. 2013; 22:14–21. PMID: 23183301.
39. Cennamo V, Luigiano C, Coccolini F, et al. Meta-analysis of randomized trials comparing endoscopic stenting and surgical decompression for colorectal cancer obstruction. Int J Colorectal Dis. 2013; 28:855–863. PMID: 23151813.

40. Ye GY, Cui Z, Chen L, Zhong M. Colonic stenting vs emergent surgery for acute left-sided malignant colonic obstruction: a systematic review and meta-analysis. World J Gastroenterol. 2012; 18:5608–5615. PMID: 23112555.
41. Zhang Y, Shi J, Shi B, Song CY, Xie WF, Chen YX. Self-expanding metallic stent as a bridge to surgery versus emergency surgery for obstructive colorectal cancer: a meta-analysis. Surg Endosc. 2012; 26:110–119. PMID: 21789642.

42. Park SJ. Tips and tricks for better endoscopic treatment of colorectal tumors: usefulness of cap and band in colorectal endoscopic mucosal resection. Clin Endosc. 2013; 46:492–494.

43. Saito Y, Emura F, Matsuda T, et al. A new sinker-assisted endoscopic submucosal dissection for colorectal cancer. Gastrointest Endosc. 2005; 62:297–301. PMID: 16046999.

44. Guda NM, Partington S, Freeman ML. Extracorporeal shock wave lithotripsy in the management of chronic calcific pancreatitis: a meta-analysis. JOP. 2005; 6:6–12. PMID: 15650279.
45. Itoi T, Yasuda I, Doi S, Mukai T, Kurihara T, Sofuni A. Endoscopic hemostasis using covered metallic stent placement for uncontrolled post-endoscopic sphincterotomy bleeding. Endoscopy. 2011; 43:369–372. PMID: 21360425.

46. Varadarajulu S, Bang JY, Hebert-Magee S. Assessment of the technical performance of the flexible 19-gauge EUS-FNA needle. Gastrointest Endosc. 2012; 76:336–343. PMID: 22817786.

47. Jang JW, Lee SS, Park DH, Seo DW, Lee SK, Kim MH. EUS-guided pancreaticogastrostomy or pancreaticoduodenostomy for symptomatic patients with pancreatic duct obstruction who are unsuitable or unsuccessful for transpapillary drainage. Endoscopy. 2011; 43:A51.

48. Cheon YK, Lee TY, Lee SM, Yoon JY, Shim CS. Longterm outcome of photodynamic therapy compared with biliary stenting alone in patients with advanced hilar cholangiocarcinoma. HPB (Oxford). 2012; 14:185–193. PMID: 22321037.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download