Abstract
A 70-year-old woman was admitted to our department with epigastric discomfort and nausea over the duration of 1 month. An esophagogastroduodenoscopy showed the presence of a 1.0×1.0 cm-sized flat lesion with central ulceration at the greater curvature side of the antrum. A biopsy demonstrated the presence of an adenocarcinoma of well differentiated, intestinal type in the stomach. Endoscopic submucosal dissection was done and the diagnosis of a composite neuroendocrine carcinoma with an adenocarcinoma of the stomach was confirmed. We report a case of a gastric composite tumor with an adenocarcinoma and neuroendocrine carcinoma confirmed by endoscopic submucosal dissection with a review of the literature.
Adenocarcinoma and neuroendocrine carcinoma are each well known to occur in the background of atrophic gastritis,1,2 but composite glandular/exocrine-endocrine carcinoma of the gastrointestinal tract is a special tumor type composed of common adenocarcinoma and the neuroendocrine component comprising of at least one third of the whole tumor area.3 These tumors, mixed exocrine-endocrine carcinomas of the stomach, are rare and mostly published as case reports.4-6
In 1987, Lewen and Appelamn first proposed a simple nomenclature for these neoplasms as follows: 1) mixed or composite tumors with an admixture of glandular and endocrine components; 2) collision tumor, where these two components are distinct and juxtaposed; 3) amphicrine cell tumors,3 composed predominantly of cells that exhibit dual endocrine and nonendocrine differentiation. Collision tumors are believed to result from two separate but adjacent neoplasms (biclonal malignant transformation), whereas composite tumors are thought to arise through a multidirectional differentiation of a single neoplasm.7,8 This case is a gastric composite tumor with an adenocarcinoma and neuroendocrine carcinoma. Adenocarcinoma is the most common malignant gastric neoplasm, whereas a gastric neuroendocrine carcinoma is relatively uncommon. In addition, a composite neuroendocrine carcinoma with an adenocarcinoma of the stomach has been rarely reported.
We present a case of gastric composite tumor displaying features of both adenocarcinoma and endocrine cell carcinoma and we discuss the histogenesis of these unusual tumors and prognostic implications of this diagnosis.
A 70-year-old woman was admitted to our department with epigastric discomfort and nausea that have lasted for 1 month. Her past medical and family histories were unremarkable. At admission, the physical examination was also unremarkable.
On blood test, there was no leukocytosis or anemia, and liver function tests were normal. The levels of tumor markers were within the normal reference range, as follows: carcinoembryonic antigen (CEA), 2.39 ng/mL (normal, <5); CA 19-9, 17.59 U/mL (normal, <37). The esophagogastroduodenoscopy showed a 1.0×1.0 cm-sized ulcerative mucosal lesion at the greater curvature side of the antrum (Fig. 1). The patient underwent endoscopic ultrasound (EUS), which revealed a 1 cm-sized isoechoic mass in the mucosal layer of the greater curvature of the antrum (Fig. 2).
The abdominal computer tomography (CT) scan examination demonstrated no evidence of metastatic regional lymph nodes.
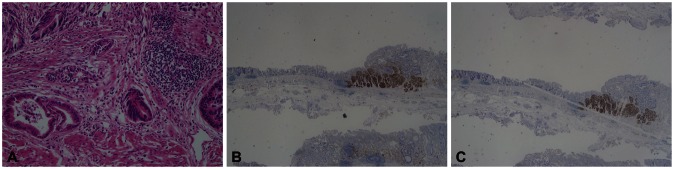
The pathologic examination of the endoscopic forceps biopsies showed a well differentiated, intestinal type adenocarcinoma. Endoscopic submucosal dissection (ESD) was performed. Microscopic sections of the resected specimen showed two components: the first component was a well-differentiated adenocarcinoma and the second was characterized as a neuroendocrine carcinoma. One area of transition between the two histological types was observed (Fig. 3).
On immunohistochemistry, the exocrine part was positive for cytokeratin p53. Neuroendocrine markers (chromogranin A, synaptophysin, and CD56) were positive in the neuroendocrine components.
The post-ESD course was uneventful and the patient was discharged on the 7th ESD day. For 12 months after the ESD, the patient has been under endoscopic, serological (CA 19-9, CEA, chromogranin A) and CT surveillance. The patient is doing well without evidence of disease recurrence.
Although the occurrence of tumors with mixed exocrine and endocrine components in the gastrointestinal tracts are very frequently reported in the appendicial region, which is also the most common location of carcinoid tumors in the gastrointestinal tract, they are very rare in the esophagus, stomach, and small and large intestines.9
In 1987, Lewen and Appelamn first proposed a simple nomenclature for these neoplasms as follows: 1) mixed or composite tumors with an admixture of glandular and endocrine components; 2) collision tumor, where these two components are distinct and juxtaposed; 3) amphicrine cell tumors,3 composed predominantly of cells that exhibit dual endocrine and nonendocrine differentiation. Collision tumors are believed to result from two separate but adjacent neoplasms (biclonal malignant transformation), whereas composite tumors are thought to arise through a multidirectional differentiation of a single neoplasm.7,8 More recently, Fujiyoshi et al.10 reclassified mixed endocrine and nonendocrine epithelial tumors by dividing the tumors into six categories: 1) neuroendocrine cells interspersed within carcinomas; 2) carcinoids (neuroendocrine tumors [NETs]) with interspersed nonendocrine cells; 3) composite glandular neuroendocrine cell carcinomas containing areas of carcinoid and conventional carcinomas; 4) collision tumors in which NETs and conventional carcinomas are closely juxtaposed, but not admixed; 5) amphicrine tumors predominantly composed of cells exhibiting concurrent neuroendocrine and nonendocrine differentiation; and 6) combinations of the previous types.
In our case, there was a gradual transitional zone between the neuroendorine carcinoma and the adenocarcinoma components. Carcinoma cells immunohistochemically stained for chromgranin A and synaptophysin were also found in some adenocarcinoma areas. Neuroendocrine component showed positively for chromgranin A and synaptophysin. According to this classification, our case could be classified as a composite glandular endocrine neuroendocrine cell carcinoma containing areas of carcinoid and conventional carcinomas.
NETs are composed of cells that are scattered throughout the mucosa of the gastrointestinal tract and express neuroendocrine markers such as chromgranin A or synaptophysin.11 The frequent transitions between the two components and similar histochemical properties further support the hypothesis of the neoplastic change of a single common precursor cell. A composite neuroendocrine carcinoma with an adenocarcinoma of the stomach is very rare, and it is very difficult to confirm the diagnosis by endoscopic findings or endoscopic biopsy. In most cases, it is possible to confirm the diagnosis by postoperative surgical specimen,8 but the present case was confirmed by ESD specimen.
The general biological behavior of a gastric composite tumor with an adenocarcinoma and a NET is unknown. A recent study suggests that gastric composite tumors have a better prognosis than common gastric adenocarcinoma,2 but due to lack of large series there are few data on the biological behavior of these tumors in comparison to adenocarcinoma. These tumors depend on adenocarcinomatous component if associated neurodenocrine component is well-differentiated, and upon the neuroendocrine component if it is poorly differentiated.12
The poor prognostic factors of NETs of the gastrointestinal tract are metastases, invasion of the muscle propria, histological differentiation, tumor size, angioinvasion, and Ki-67 index (>30%).13 In most cases, NETs were diagnosed metastatic to liver or bone, with extension to either serosa and/or lymph nodes at the time of diagnosis, so their prognosis is poor.14 In general, surgery is the choice of treatment for NETs of the gastrointestinal tract. Postoperative chemotherapy is recommended in advanced cases.15 In our case, because any distant metastasis or lymph nodes were not found by EUS and abdominal CT, ESD was performed. The resected specimen measured 3.0×2.8 cm. Deep and lateral margin was free from tumor. There were an adenocarcinoma component measuring 1.4×1.3 cm, and a neuroendocrine component measuring 0.6×0.5 cm, confined within the submucosa (70/980 µm, invasion depth/dissected submucosal depth, upper 1/3 of submucosa), well-differentiated, angioinvasion (-), and Ki-67 index (<2%).
According to the criteria for assessing the prognosis of NETs of the gastrointestinal tract,16 the suspected biological behavior was not poor. NETs were essentially benign lesions in this case, therefore treatment was determined by the adenocarcinoma of the stomach. Because the patient was old and didn't want surgical treatment, a surgical treatment did not follow the ESD in this patient.
In conclusion, gastric composite tumors are rare; all cases involving the gastrointestinal tract have been reported as case reports, so their prognosis and biological behavior have not been fully clarified. However, as more of such rare tumors with a spectrum of morphological combinations are reported, their prognosis and biological behavior will become better understood in the near future.
References
1. Reis-Filho JS, Schmitt FC. Amphicrine gastric carcinoma. Arch Pathol Lab Med. 2001; 125:1513–1514. PMID: 11698020.

2. Yamashina M, Flinner RA. Concurrent occurrence of adenocarcinoma and carcinoid tumor in the stomach: a composite tumor or collision tumors? Am J Clin Pathol. 1985; 83:233–236. PMID: 3969962.

3. Lewin K. Carcinoid tumors and the mixed (composite) glandular-endocrine cell carcinomas. Am J Surg Pathol. 1987; 11(Suppl 1):71–86. PMID: 3544888.

4. Strofilas A, Dalianoudis IG, Lagoudianakis EE, et al. Collision tumour of the stomach with a cancer to cancer metastasis: a case report. Cases J. 2008; 1:63. PMID: 18662400.

5. Morishita Y, Sugitani M, Sheikh A, Nemoto N, Fujii M, Takayama T. Collision tumor of the stomach: a rare case of an adenocarcinoma and carcinoid tumor. Arch Pathol Lab Med. 2005; 129:407–409. PMID: 15737041.

6. Kim EY, Park KC, Kwon JG. A case of double primary cancer: early gastric adenocarcinoma associated with adenocarcinoma and carcinoid. Korean J Gastroenterol. 2003; 42:533–538. PMID: 14695711.
7. Liu SW, Chen GH, Hsieh PP. Collision tumor of the stomach: a case report of mixed gastrointestinal stromal tumor and adenocarcinoma. J Clin Gastroenterol. 2002; 35:332–334. PMID: 12352297.
8. Fukui H, Takada M, Chiba T, et al. Concurrent occurrence of gastric adenocarcinoma and duodenal neuroendocrine cell carcinoma: a composite tumour or collision tumours? Gut. 2001; 48:853–856. PMID: 11358908.
9. Levendoglu H, Cox CA, Nadimpalli V. Composite (adenocarcinoid) tumors of the gastrointestinal tract. Dig Dis Sci. 1990; 35:519–525. PMID: 2180655.

10. Fujiyoshi Y, Kuhara H, Eimoto T. Composite glandular-endocrine cell carcinoma of the stomach. Report of two cases with goblet cell carcinoid component. Pathol Res Pract. 2005; 200:823–829. PMID: 15792127.

11. Granberg D, Wilander E, Stridsberg M, Granerus G, Skogseid B, Oberg K. Clinical symptoms, hormone profiles, treatment, and prognosis in patients with gastric carcinoids. Gut. 1998; 43:223–228. PMID: 10189848.

12. Volante M, Rindi G, Papotti M. The grey zone between pure (neuro)endocrine and non-(neuro)endocrine tumours: a comment on concepts and classification of mixed exocrine-endocrine neoplasms. Virchows Arch. 2006; 449:499–506. PMID: 17033797.

13. Klöppel G, Perren A, Heitz PU. The gastroenteropancreatic neuroendocrine cell system and its tumors: the WHO classification. Ann N Y Acad Sci. 2004; 1014:13–27. PMID: 15153416.
14. Rindi G, Luinetti O, Cornaggia M, Capella C, Solcia E. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology. 1993; 104:994–1006. PMID: 7681798.

15. Gilligan CJ, Lawton GP, Tang LH, West AB, Modlin IM. Gastric carcinoid tumors: the biology and therapy of an enigmatic and controversial lesion. Am J Gastroenterol. 1995; 90:338–352. PMID: 7872269.
16. Klöppel G, Anlauf M. Epidemiology, tumour biology and histopathological classification of neuroendocrine tumours of the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2005; 19:507–517. PMID: 16183524.

Fig. 1
The esophagogastroduodenoscopy finding. The picture showed a 1.0×1.0-cm-sized ulcerative mucosal lesion at the greater curvature side of the distal antrum.
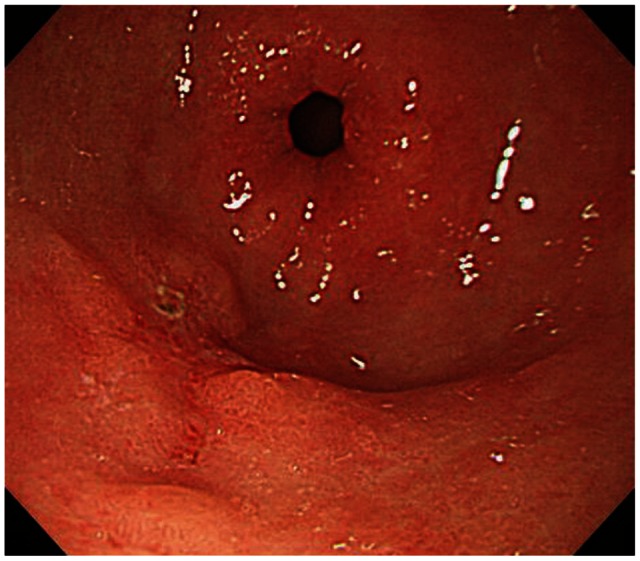
Fig. 2
The patient underwent endoscopic ultrasound. The picture revealed a 1-cm-sized isoechoic mass in the mucosal layer of the greater curvature of the distal antrum.
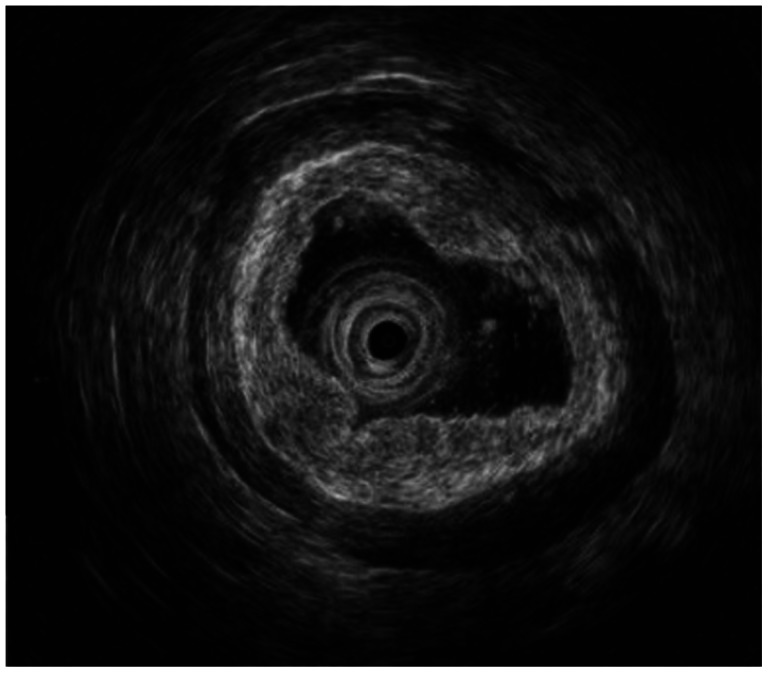
Fig. 3
Pathologic findings. (A) It shows a well-differentiated adenocarcinoma and a neuroendocrine carcinoma. One area of transition between the two histologic types was observed (H&E stain, ×200). (B) The tumor cells with endocrine differentiation are highlighted by CD 56 stain, but adenocarcinoma shows no immunoreactivity (CD 56 stain, ×40). (C) The tumor cells with endocrine differentiation are highlighted by synaptophysin stain, but adenocarcinoma shows no immunoreactivity (synaptophysin stain, ×40).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download