Abstract
Endoscopic treatment for non-variceal upper gastrointestinal bleeding has evolved over decades. Injection with diluted epinephrine is considered as a less than adequate treatment, and the current standard therapy should include second modality if epinephrine injection is used initially. Definitive hemostasis rate following mono-therapy with either thermo-coagulation or hemo-clipping compares favorably with dual therapies. The use of adsorptive powder (Hemo-spray) is a promising treatment although it needs comparative studies between hemospray and other modalities. Stronger hemo-clips with better torque control and wider span are now available. Over-the-scope clips capture a large amount of tissue and may prove useful in refractory bleeding. Experimental treatments include an endoscopic stitch device to over-sew the bleeding lesion and targeted therapy to the sub-serosal bleeding artery as guided by echo-endoscopy. Angiographic embolization of bleeding artery should be considered in chronic ulcers that fail endoscopic treatment especially in elderly patients with a major bleed manifested in hypotension.
Acute upper gastrointestinal (GI) bleeding is one of commonest medical emergencies. The condition is associated with a high death rate. This short review aims to outline the current standards in the endoscopic treatment of acute upper GI bleeding from a non-variceal cause and to provide an overview of the new endoscopic treatments being evaluated in pilot clinical trials and in experimental settings.
It has been almost four decades since the first report of injection therapy to bleeding peptic ulcers.1 Endoscopic hemostatic treatment has continued to evolve. It is now clear that injection with diluted epinephrine is a suboptimal treatment. Injection therapy works mostly by volume tamponade on the artery. It does not induce vessel thrombosis. Soehendra proposed the original concept of combination therapy-pre-injection with diluted epinephrine followed by targeted treatment of the artery. Calvet et al.2 summarized findings from 16 randomized studies that compared epinephrine injection alone to epinephrine injection followed by an added treatment of the vessel. The meta-analysis consisted of 1,673 patients. Adding a second modality was shown to reduce further bleeding from 18.4% to 10.6% (Peto odds ratio [OR], 0.53; 95% confidence interval [CI], 0.40 to 0.69), emergency surgery from 11.3% to 7.6% (OR, 0.64; 95% CI, 0.46 to 0.90), and importantly death from 5.1% to 2.6% (OR, 0.51; 95% CI, 0.31 to 0.84). In another pooled analysis of 2,472 patients, Marmo et al.3 compared mono- to dual therapy and found that dual endoscopic therapy reduced the risk of recurrent bleeding (OR, 0.59; 95% CI, 0.44 to 0.88; p<0.001), risk of emergency surgery (OR, 0.66; 95% CI, 0.49 to 0.89; p=0.03), and showed a trend toward a reduction in the risk of death (OR, 0.68; 95% CI, 0.46 to 1.02; p=0.06) (Fig. 1).3 In subgroup analysis, dual therapy was significantly superior to injection therapy alone for all the outcomes considered, but failed to demonstrate that any combination of treatments is better than either mechanical therapy alone or thermal therapy alone. These two reviews suggest that injection with diluted epinephrine should only be used to stop or slow down bleeding for a clear view of the artery. Either hemo-clipping or thermo-coagulation to the artery should follow. When an artery (or a protuberant discoloration) can be seen without injection, one can proceed to hemo-clipping or thermo-coagulation.
The choice between hemoclips and thermo-coagulation is not clear. Often endoscopists switch from one to another if hemostasis is not successful in the first instance. We summarized randomized controlled trials that compared thermocoagulations to hemoclips with or without injections.4 The rate of definitive hemostasis following thermo-coagulation or clips was almost identical (81.5% vs. 81.2%; relative risks, 1.00; 95% CI, 0.77 to 1.31). Either modality is efficacious in securing hemostasis. It is a common practice to start with the use of hemoclips. The application of hemoclips would be ideal where there is a clear vessel head or 'protuberance.' We have found application of hemoclips difficult in the posterior wall of duodenal bulb, and the lesser curve of the stomach. Tangential application of hemoclips or their applications with a scope in a retroflexed position often fail. Jaws of hemoclips may not grip into ulcer base that is hard and fibrotic. In these circumstances, we prefer the use of contact thermo-coagulation and specifically the use of a 3.2 mm heater probe. We occasionally use a side viewing duodenoscope for better positioning to deliver treatment. The use of a thermal device is somewhat easier when compared to that of hemoclips. Mechanical compression of the artery is the critical component of the heater probe treatment. The artery is compressed firmly to stop blood flow followed by wielding of the arterial walls by thermal energy (concept of co-aptive thermo-coagulation). We use an energy setting of 30 Joules. To each station, a consecutive four pulses are applied lasting for approximately 8 seconds. The probe is gently 'floated' off the foot print by activating the irrigation ports.
The Hemospray powder (Haemospray, TC-325; Cook Medical Inc., Winston-Salem, NC, USA) is a highly adsorptive powder made of proprietary mineral blend. When in contact with blood, the powder becomes cohesive and forms a stable mechanical plug that covers the bleeding site. It is approved by the Food and Drug Administration of USA for external traumatic uses. We reported its first GI use in 20 patients with Forrest I bleeding peptic ulcers.5 During endoscopy, the powder was power-sprayed onto the bleeding site with the use of a delivery catheter connected to a carbon-dioxide canister. Short bursts of powder were applied until cessation of bleeding (Fig. 2). The site was observed for a further 5 minutes. We were able to achieve hemostasis in 19 of 20 patients. One patient with a large angular notch ulcer had refractory bleeding and eventually underwent visceral angiography which showed a pseudo-aneurysm to the left gastric artery. The aneurysm was then coiled. No significant recurrent bleeding was seen in 19 treated patients. No systemic effect from Hemospray was observed as the powder was not absorbed. The powder is a promising new hemostatic agent. It appeals to endoscopists for its simplicity in use. More data, especially comparative ones, are required to determine the true merits of this powder among other available treatment modalities.
Hachisu et al.6 first reported the use of hemoclips in 1989. There are several clips available in the market including the Quick clips by Olympus (Tokyo, Japan), Resolution clip (Boston Scientific Co., Natick, MA, USA), the three pronged triclip and recently a large Instinct clip (Cook Medical Inc., Winston-Salem, NC, USA). The largest clip opens to 18 mm in width. An ideal clip should be strong enough to grip into tissue firmly for approximation, able to open and close before its final deployment and have good torque control for its optimal orientation and positioning. The use of hemoclips in a side-viewing endoscope is often a problem. There have been few comparative studies on different types of clips. In a randomized study of 100 patients, triclips were compared to hemoclips in securing ulcer hemostasis. Initial hemostasis was higher with the use of hemoclips (94% vs. 76%).7 The jaws in the three pronged designs are often not strong enough in fibrotic ulcers. The trial reinforces our impression that one of the key elements to successful hemostasis is the strength of the jaws. A larger amount of captured tissue is often desirable.
An over-the-scope clip (Ovesco Endoscopy, Tübingen, Germany) is being marketed for use in closure of GI defects and tissue approximation. A nitinol clip is pre-mounted on a cap which is then attached to the tip of an endoscope. Tissue is drawn into the cap either by suction, or by the use of a specially designed twin grasper for tissue approximation or an anchor. The design captures a large amount of tissue and is more secure than other clips in experimental setting. The system is being used in complex GI bleeding.8 A single clip suffices for most circumstances and therefore the procedure is shorter when compared to multiple applications of hemoclips.
Endoscopic suturing is close to a stitch applied during surgery. It is arguably the most secure form of hemostasis. An endoscopic suturing system Eagle Claw (Olympus) was developed and bench tested first in an Erlangen model and then a live porcine model of bleeding ulcer from an artery of >2 mm in size. In survival heparinized pig models, the device was effective in stopping major bleeding.9,10 Its earlier version required extra-corporeal knots being tied and pushed into place by a knotting cap. Based on a similar concept, an endoscopic suturing system Overstitch (Appollo Endosurgery Inc., Austin, TX, USA) is being marketed for use in endoscopic tissue approximation. The system has a curve needle with a detachable tip that carries either a 2.0 or 3.0 suture. The curve needle is driven through mucosa. Its tip is then detached and needle exits through mucosa. The suture is 'tied' down using a cinching device. With multiple needle passes, the system is capable of deploying multiple running and interrupted stitches with a single insertion of an endoscope. A clinical trial to evaluate its use in patients with bleeding peptic ulcers is being planned (Fig. 3).
In an ex vivo model that used canine mesenteric arteries, the use of 3.2 mm contact thermal device was shown to consistently seal arteries of up to 2 mm in diameter.11 A 2 mm artery is often cited as the limit to endoscopic therapy. Swain et al.12 studied 27 gastrectomy specimens from patients with bleeding gastric ulcers using thin barium angiography. Mean external diameter of bleeding artery was 0.7 mm (0.1 to 1.8). Aneurysmal dilatation of the eroded artery was seen in 14 of 27 arteries. Thirteen arteries were technically outside stomach wall and were sub-serosal in disposition. It is not clear if endoscopic therapy was carried out in this series of patients who came to eventual surgery. In an abstract, Lai and Swain13 reported post-mortem findings of patients after fatal bleeding and found a mean arterial external diameter of 3.45 mm. In a systematic review of published studies on factors that would predict failure to endoscopic treatment, Elmunzer et al.14 found large ulcers (2 cm in size) located at bulbar duodenum or the lesser curvature of the stomach, active bleeding, hemodynamic instability, and presence of comorbid illnesses as common factors. These studies suggest that patients with severe bleeding manifested in shock and with large ulcers that are likely to erode into large arterial complexes (gastro-duodenal or left gastric artery) may benefit from angiographic embolization in addition to endoscopic treatment.
There have been significant advances in interventional radiology over the past decades. These include the use of microcatheter techniques and newer coil devices for embolization. Currently we are investigating the use of angiographic embolization as an adjunct to endoscopic treatment. Berne and Rosoff15 provided an account on the anatomy of bleeding gastro-duodenal artery. There is often a transverse pancreatic branch before the confluence of superior pancreatico-duodenal artery and right gastroepiploic artery. The technique of gastroduodenal embolization is also known as the sandwich technique which involves packing of the confluence between superior pancreatico-duodenal artery and the right gastroepiploic artery, followed by gelfoam plugs to block collaterals and then more coils in the proximal portion (Fig. 4).
There have been anecdotal reports of experimental use of endoscopic ultrasonography (EUS) guided puncture of bleeding sub-serosal arteries in live porcine models of a bleeding gastric ulcer.16 Elmunzer et al.16 reported the use of a forward viewing echoendoscope for this purpose. Thomson's group has experimented over EUS puncture of celiac artery and injection of biodegradable polymer to temporarily arrest bleeding.17 There has not been a report of such an approach in clinical use yet.
Current endoscopic modalities provide durable hemostasis in over 85% of cases. With continued improvement in mechanical devices and adjunctive use of angiographic embolization, it is likely that the rate of continued or recurrent bleeding will continue to decline translating into reduced patient deaths from the condition.
References
1. Soehendra N, Werner B. New technique for endoscopic treatment of bleeding gastric ulcer. Endoscopy. 1977; 8:85–87. PMID: 862577.

2. Calvet X, Vergara M, Brullet E, Gisbert JP, Campo R. Addition of a second endoscopic treatment following epinephrine injection improves outcome in high-risk bleeding ulcers. Gastroenterology. 2004; 126:441–450. PMID: 14762781.

3. Marmo R, Rotondano G, Piscopo R, Bianco MA, D'Angella R, Cipolletta L. Dual therapy versus monotherapy in the endoscopic treatment of high-risk bleeding ulcers: a meta-analysis of controlled trials. Am J Gastroenterol. 2007; 102:279–289. PMID: 17311650.

4. Sung JJ, Tsoi KK, Lai LH, Wu JC, Lau JY. Endoscopic clipping versus injection and thermo-coagulation in the treatment of non-variceal upper gastrointestinal bleeding: a meta-analysis. Gut. 2007; 56:1364–1373. PMID: 17566018.

5. Sung JJ, Luo D, Wu JC, et al. Early clinical experience of the safety and effectiveness of Hemospray in achieving hemostasis in patients with acute peptic ulcer bleeding. Endoscopy. 2011; 43:291–295. PMID: 21455870.

6. Hachisu T, Miyazaki S, Hamaguchi K. Endoscopic clip-marking of lesions using the newly developed HX-3L clip. Surg Endosc. 1989; 3:142–147. PMID: 2814777.

7. Lin HJ, Lo WC, Cheng YC, Perng CL. Endoscopic hemoclip versus triclip placement in patients with high-risk peptic ulcer bleeding. Am J Gastroenterol. 2007; 102:539–543. PMID: 17100962.

8. Kirschniak A, Subotova N, Zieker D, Königsrainer A, Kratt T. The Over-The-Scope Clip (OTSC) for the treatment of gastrointestinal bleeding, perforations, and fistulas. Surg Endosc. 2011; 25:2901–2905. PMID: 21424197.

9. Hu B, Chung SC, Sun LC, et al. Developing an animal model of massive ulcer bleeding for assessing endoscopic hemostatic devices. Endoscopy. 2005; 37:847–851. PMID: 16116536.

10. Chiu PW, Hu B, Lau JY, Sun LC, Sung JJ, Chung SS. Endoscopic plication of massively bleeding peptic ulcer by using the Eagle Claw VII device: a feasibility study in a porcine model. Gastrointest Endosc. 2006; 63:681–685. PMID: 16564872.

11. Johnston JH, Jensen DM, Auth D. Experimental comparison of endoscopic yttrium-aluminum-garnet laser, electrosurgery, and heater probe for canine gut arterial coagulation. Importance of compression and avoidance of erosion. Gastroenterology. 1987; 92(5 Pt 1):1101–1108. PMID: 3493938.
12. Swain CP, Storey DW, Bown SG, et al. Nature of the bleeding vessel in recurrently bleeding gastric ulcers. Gastroenterology. 1986; 90:595–608. PMID: 3943691.

13. Lai KC, Swain CP. The size of vessel in patients dying from bleeding gastric ulcer. Gastroenterology. 1993; 104(4 Suppl):A202.
14. Elmunzer BJ, Young SD, Inadomi JM, Schoenfeld P, Laine L. Systematic review of the predictors of recurrent hemorrhage after endoscopic hemostatic therapy for bleeding peptic ulcers. Am J Gastroenterol. 2008; 103:2625–2632. PMID: 18684171.

15. Berne CJ, Rosoff L. Peptic ulcer perforation of the gastroduodenal artery complex: clinical features and operative control. Ann Surg. 1969; 169:141–144. PMID: 4883483.
16. Elmunzer BJ, Pollack MJ, Trunzo JA, et al. Initial evaluation of a novel, prototype, forward-viewing echoendoscope in a porcine arterial bleeding model (with video). Gastrointest Endosc. 2010; 72:611–614. PMID: 20630519.

17. Shaikh SN, Ryou M, Azagury DE, Thrompson CC. New techniques in gastrointestinal hemostasis. Gastrointest Endosc. 2010; 71:AB100.
Fig. 1
A forest plot of a meta-analysis that compared mono- to dual endoscopic therapies in the treatment of non-variceal upper gastrointestinal bleeding (modified from Marmo et al. Am J Gastroenterol 2007;102:279-289).3 CI, confidence interval.

Fig. 2
Hemospray treatment of a bleeding gastric ulcer; short bursts of powder were sprayed onto the ulcer (A-C). On follow-up endoscopy at day 3, a flat pigment was seen on the ulcer crater (D).
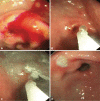
Fig. 3
An actively bleeding duodenal ulcer was over-sewn with the overstitch device (A-D). Three stitches were applied.
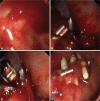
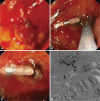
Fig. 4
A large bulbar ulcer that failed hemoclipping (A) was treated by thermo-coagulation using a 3.2 mm heater probe (B, C). The gastroduodenal artery was then coiled during angiography (D). The picture depicts a microcatheter in the common hepatic artery and a larger Simmon's catheter in the celiac artery. The hemoclips provide landmark to the site for empirical coiling. Coils are first dropped distal to the bleeding point. Gelfoams are then used to block collateral branches. Further coils are then added to the proximal portion of the gastro-duodenal artery.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download