Abstract
Since scalp hair loss has increased recently even in young people, seriously affecting individual's quality of life, the hair growth-stimulating effects of Laminaria japonica extract (LJE) and Cistanche tubulosa extract (CTE) were investigated. After confirming anagen phase of follicles under shaving, male C57BL/6 mice were dermally applied with 3% Minoxidil or orally administered with the combinations of LJE and CTE for 21 days. Minoxidil promoted the hair regrowth and increased γ-glutamyl transpeptidase (γ-GTP) and alkaline phosphatase (ALP) activities. In addition, Minoxidil up-regulated epidermal growth factor (EGF) and vascular endothelial growth factor (VEGF) levels. Co-administration of LJE and CTE at 54 mg/kg LJE plus 162 mg/kg CTE exerted synergistic promoting effects on the hair regrowth, comparable to 3% Minoxidil. LJE preferentially enhanced ALP activity, while CTE increased both γ-GTP and ALP activities as well as EGF and VEGF expressions. In vivo air pouch inflammation model, carrageenan-induced vascular exudation and increased nitric oxide and prostaglandin E2 concentrations in the exudates were synergistically suppressed by co-administration of LJE and CTE. In addition, inflammatory cell infiltration was substantially inhibited by the combinational treatment. The results suggest that combinational oral treatment with LJE and CTE in appropriate doses and ratios prevent hair loss and improve alopecia, which might be in part mediated by their anti-inflammatory activities.
Recently, scalp hair loss has been increasing even in young people, seriously affecting individual's quality of life. Although baldness is not one of the health problems, it may cause profound psychologically-uncomfortable social stress. Therefore, it is of great interest to develop novel preventive and/or therapeutic materials for hair loss and regrowth. In spite of the billion-dollar market, there are a few currently-effective products available [1].
Hair follicles remodel themselves during cycling periods of growth (anagen), regression (catagen), resting (telogen), and shedding (exogen) [2]. During catagen, lots of the follicular cells undergoes programmed cell death (apoptosis) [3], reducing the follicle size as the follicles enter telogen. Activation of rarely-cycling epithelial stem cells located in the bulge region of the follicles renders follicular regeneration at the onset of the next anagen phase [4]. Stem cell progeny forms a new follicular matrix during early anagen, and the hair shaft and inner root sheath are derived from these relatively-undifferentiated matrix cells [5]. It is well known that the size of the hair follicle and the duration of anagen phase govern the size (diameter) and length of the hair shaft, respectively. This cyclic growth of hair is regulated by diverse growth factors.
Minoxidil, finasteride, and propecia are commercially available to slow hair loss and to facilitate hair regrowth. Minoxidil as a vasodilator exerted a side-effect to facilitate hair growth [6] which may be due to its nutrient-supplying and potassium channel-opening activities [7,8]. Minoxidil is effective for androgenic alopecia, among other baldness treatments, although its effectiveness disappears within months after discontinuation of treatment. By comparison, finasteride and propecia, therapeutics of benign prostatic hyperplasia and prostate cancer, have been registered in many countries for androgenetic alopecia (male-pattern baldness). Finasteride and propecia are synthetic anti-androgens, inhibiting type II 5-α reductase, an enzyme that converts testosterone to dihydrotestosterone (DHT) in the papilla cells at the bottom of the hair follicles [9].
Laminaria japonica, Undaria pinnatifida, and Cladosiphon okamuranus, widely distributed in many countries, have been used as therapeutics in Oriental medicine for a long period. In previous studies, fucoidan, a sulfate polysaccharide complex, had been extracted and identified from the seaweeds, and confirmed to possess anti-oxidative, anti-coagulative, anti-cancer, and anti-inflammatory activities [10,11]. Thereafter, beneficial effects of fucoidan on inflammatory diseases, ischemia, immune dysfunction, and tumors have been demonstrated [12,13]. On the other hand, Cistanche tubulosa extract (CTE) has also been widely used for traditional medication in China. CTE improves blood flow by lowering blood cholesterol level [14]. Recently, it was reported that CTE decreased the production of tumor-necrosis factor-α (TNF-α) and interleukin-4 (IL-4) that are major factors for nitric oxide (NO) production in inflammatory pathway [15]. We also demonstrated that fucoidan from Laminaria japonica extract (LJE) and CTE synergistically attenuated inflammation in vitro and in vivo [16]. Notably, it has been demonstrated that there was a close association of skin (especially lacrimal gland) inflammation with alopecia [17]. Thus, anti-inflammatory aspects of alopecia treatment are emerging [18].
Based on the improving activities on blood flow and inflammation, we investigate the combinational efficacy of LJE and CTE on hair growth and underlying mechanism(s), in comparison with 3% Minoxidil (Hyundai Pharmaceutical, Seoul, Korea), a well-known hair growth enhancer.
LJE and CTE were obtained from Misuba RTech Co., Ltd. (Asan, Korea). Dried powder of Laminaria japonica was extracted in purified water at 60℃ for 24 hours, filtered through an 0.45 µm membrane, disinfected, and spray-dried. Cistanche tubulosa was extracted in 80% ethanol, disinfected, concentrated, and dried. LJE and CTE were kept at 4℃, mixed (1:3) and dissolved in purified water before use, and orally administered in a volume of 5 mL/kg.
Male C57BL/6 mice for hair growth study and male ICR mice for inflammation study were purchased from the Daehan Biolink (Eumseong, Korea), and housed in a room with constant environmental conditions (23±2℃; 45-65% relative humidity; 12-hour light-dark cycle; 150-300 lux brightness). Pellet feed and purified water were available ad libitum. All the animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Chungbuk National University (CBNU), Korea, and conducted according to the Standard Operation Procedures (SOP) of the Laboratory Animal Research Center, CBNU.
After confirming anagen phase by observing homogeneously-pink skin color in the back skin of C57BL/6 mice (6 weeks old, n=8/group) after clipping in telogen phase, the hair was shaved [1,19,20]. From the next day, mice without visible scratches were selected, and orally treated with LJE (18 or 54 mg/kg), CTE (54 or 162 mg/kg), or their mixture (18+54 or 54+162 mg/kg) once a day for 21 days. Low doses of LJE (18 mg/kg) and CTE (54 mg/kg) were from estimated human doses of 100 mg/body (70 kg) and 300 mg/body after surface area translation of body weight (Km=37 for human/Km=3 for mouse), respectively. High doses of LJE and CTE were set at 3 folds, alone or in combination, to assess maximum synergistic effects [16]. Minoxidil (3%, 0.2 mL) was topically applied once a day on the shaved skin in the same schedule with LJE/CTE treatment.
On days at 3-day intervals after shaving and during treatment, the mice were lightly anesthetized with diethyl ether and photographs were taken on their back sides. The ratio of hair regrowth (regrown area/shaved area) on the photographs was analyzed using a computerized image analysis system (Imageinside, Daejeon, Korea).
On days 14 and 21, the mice were sacrificed by deep anesthesia with diethyl ether, and their dorsal skins were removed [1]. The dorsal skin was cut into two parts. Formalin-fixed, paraffin-embedded tissue sections (5 µm in diameter) of one part were stained with hematoxylin-eosin to examine the number of hair follicles and the histological findings. The other part of the tissue was frozen in liquid nitrogen for enzyme assay and immunoassay.
Skin tissue was homogenized in 4 volumes of phosphate buffer (pH 7.4). The homogenate was centrifuged at 3,000 rpm for 20 min, and the supernatant was used for assay of γ-glutamyl transpeptidase (γ-GTP) and alkaline phosphatase (ALP) activities using p-nitrophenol phosphate (PNPP)-DEAV as substrate [1,21].
ICR mice (7 weeks old, n=8/group) were orally treated with LJE (18 or 54 mg/kg), CTE (54 or 162 mg/kg), or their mixture (18+54 or 54+162 mg/kg) once a day for 7 days [16]. On the 1st day of LJE and CTE treatment, the mice were subcutaneously injected with 10 mL of sterile air into the back side to form a pouch [16,23,24]. After 2 and 5 days, the pouch was re-injected with 5 mL of air. One hour after the final LJE and CTE treatment, 1 mL of carrageenan (1% in saline; Sigma-Aldrich, St. Louis, USA) or its vehicle (saline) was injected into the pouch.
The air pouch was washed with 1 mL of cold saline 6 hours after carrageenan injection, and the net volume of lavage fluid was recorded [16]. Total numbers of inflammatory cells including neutrophils, monocytes, and lymphocytes were determined using a coulter counter. The concentrations of NO and prostaglandin E2 (PGE2) were determined by Griess reagent (Sigma-Aldrich) and enzyme immunoassay (EIA) using a Correlate-EIA kit (Assay Designs, Ann Arbor, USA), respectively [16].
Application of Minoxidil (3%), a reference material, markedly facilitated the hair growth of C57BL/6 mice, exhibiting a near-full coverage (92.4%) of the shaved area (Figures 1, 2B) compared to the 29.2% in normal growth (Figures 1, 2A) on day 21. Although less effective than Minoxidil application, oral treatment of LJE also facilitated the hair growth, leading to 33.5 and 69.1% coverage at 18 and 54 mg/kg, respectively (Figures 1, 2C, 2D). CTE was also effective in enhancing the hair growth, reaching 73.6 and 80.4% coverage at 54 and 162 mg/kg, respectively (Figures 1, 2E, 2F). Notably, combinational therapy with high doses of LJE (54 mg/kg) and CTE (162 mg/kg) remarkably enhanced the hair growth from day 12, comparable to the effect of Minoxidil (Figures 1, 2H), although low doses of LJE (18 mg/kg) and CTE (54 mg/kg) exert a synergistic effect only at early phase (Figures 1, 2G).
Compared to normal hair follicles (Figure 3A), Minoxidil application improved the integrity of hair follicles, showing bigger follicles and thicker hair shafts (Figure 3B). Such effects were also found in the skins of mice treated with LJE and/or CTE (Figures 3C-3H). Especially, the hair follicle-strengthening efficacy of combinational treatment of LJE and CTE was comparable to that of Minoxidil.
Minoxidil application increased the activity of γ-GTP that induces hair follicles to anagen phase by 90-120% on days 14 and 21, compared to the level of normal skin (Figure 4). LJE (54 mg/kg) also enhanced the enzyme activity, although there were high variations. CTE exerted similar effects, significantly enhancing the γ-GTP activity on day 14. Interestingly, combinational treatment of LJE and CTE further increased the enzyme activity higher than that when the extracts were used alone.
ALP activity, a marker of bone growth and angiogenesis, significantly increased in the skin on days 14 and 21 following topical application of Minoxidil (Figure 5). LJE and CTE also increased the ALP activity after oral administration in a dose-dependent manner. Notably, the enzyme activity was synergistically enhanced by combinational treatment with LJE and CTE.
In the analysis of EGF, a well-known hair growth factor, Minoxidil application enhanced the level of EGF 3.5 folds of normal control on both days 14 and 21 (Figure 6). LJE (18 mg/kg) increased EGF only on day 21, while CTE (162 mg/kg) greatly up-regulated its production on days 14 and 21. It is of interest to note that the combinational treatment of LJE and CTE even at low doses exhibited marked synergistic effects.
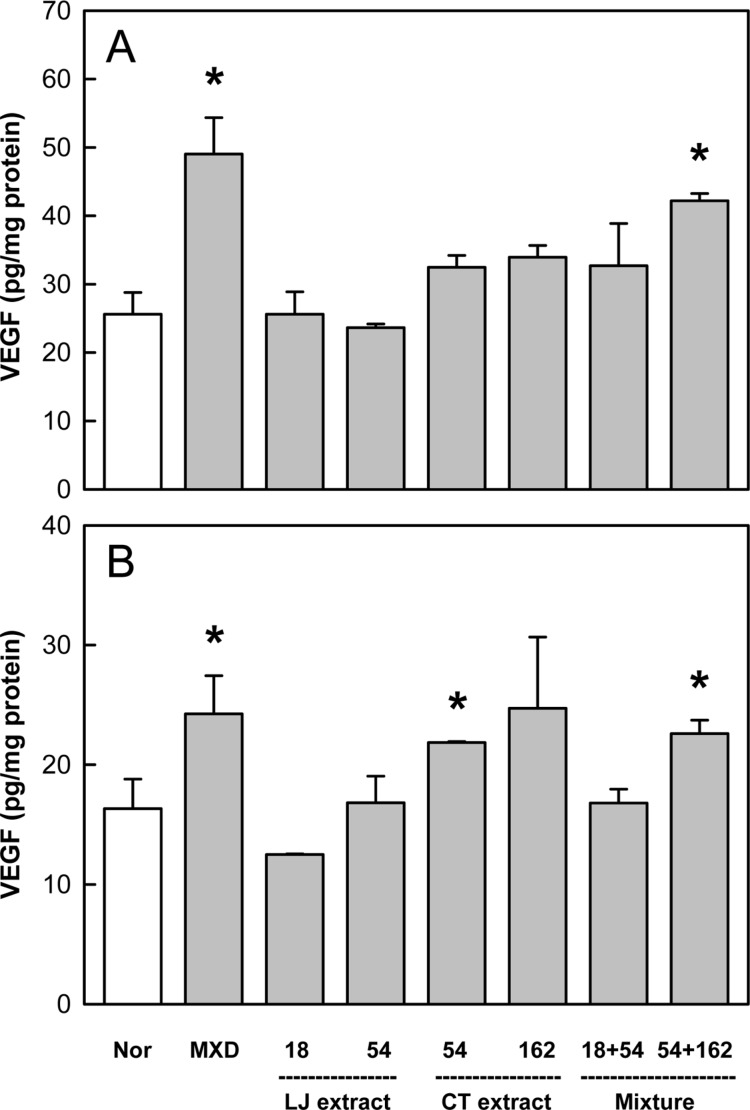
Minoxidil treatment also increased the level of VEGF by 50-80% above the control value (Figure 7). Although LJE was ineffective, CTE exerted remarkable enhancing effects. In addition, combinational therapy of LJE and CTE displayed a synergistic activity at early phase on day 14.
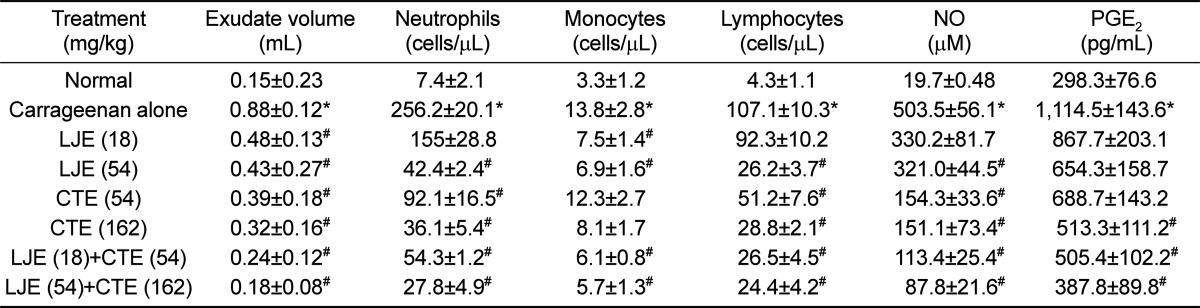
Injection of carrageenan into ICR mouse air pouch increased the exudate volume within the pouch 6 times the control level (Table 1). Such increased exudation was significantly attenuated by treatment with LJE (18 and 54 mg/kg) or CTE (54 and 162 mg/kg). Notably, combinational treatment with LJE and CTE further decreased the exudate volume, compared to the individual effects. The number of inflammatory cells was drastically increased by carrageenan injection: 34.6, 4.2, and 24.9 folds control levels for neutrophils, monocytes, and lymphocytes, respectively (Table 1). However, LJE and CTE significantly reduced the inflammatory cells in dose-dependent manners, in which LJE was somewhat superior to CTE. Expectedly, synergistic effects in the inflammatory cell infiltration were attained by co-administration of LJE and CTE.
In parallel with the inflammatory cell infiltration in the pouch, NO and PGE2 contents in the pouch also drastically increased following carrageenan injection (Table 1). In comparison with the mild effect of LJE, CTE markedly lowered the NO concentration. Combinational treatment of LJE and CTE further decreased the NO level in a dose-dependent manner. In a similar manner, the increased PGE2 level was synergistically suppressed by the combinational therapy of LJE and CTE (Table 1).
Human head has an average of 100,000 hair follicles. During lifetime, each follicle grows and sheds about 20 times, showing daily hair loss of 100 strands [25]. Hair loss including alopecia and baldness is a distressing condition for men and women especially in modern society. Therefore, it is of great importance to develop new therapies that prevent hair loss and enhance hair regrowth. In this respect, alternative medicine has attracted investigators' interest.
Aging-related morphological, histochemical, and biochemical aspects of male-pattern baldness have been revealed by a series of studies on stump-tailed macaques. In the male-pattern baldness, telogen phase was prolonged, while anagen phase shortened. Thus, it is considered that for improvement of hair loss, maintenance of hair follicle elongation that occurs from early anagen to mid anagen is very important [26]. In the present study, we demonstrated the hair growth-facilitating activity of a combinational treatment LJE and CTE. Most importantly, the combinational prescription of LJE (54 mg/kg) and CTE (162mg/kg) exhibited an excellent hair regrowth activity, in spite of oral administration of the extracts. Notably, the effect of combinational therapy was comparable to that of dermal application of a high concentration (3%) of a purified compound Minoxidil, although the concentrations of active ingredients of LJE and CTE in the skin after oral administration were not confirmed.
In the cycling mouse skin, γ-GTP activity is pronounced during anagen, but greatly diminished during telogen [27]. In the skin, the enzyme exists exclusively in the outer and inner root sheaths of hair follicles below the level of the sebaceous glands in which γ-GTP is absent [27]. By comparison, ALP is prominently expressed in the pilosebaceous units, and the activity increases in the area of hair regrowth. ALP as an angiogenic factor improves nutritional supply to dermal papillae, and thereby induces thickness during anagen [28]. However, it was confirmed that the ALP activity decreased in the alopecic skin [21]. In the mechanistic study, the reference compound Minoxidil markedly enhanced the activity of γ-GTP and ALP, implying that Minoxidil facilitates hair growth not only by inducing hair follicles to anagen phase, but also by improving blood flow via angiogenesis. Since LJE increased ALP activity, it is proposed that LJE exhibits a beneficial effect on the hair growth through its angiogenic potential, as inferred from a previous study that fucoidan, an active ingredient of LJE, has a blood flow-improving activity [29]. By comparison, CTE enhanced both the γ-GTP and ALP activities, suggestive of its effects on the anagen phase induction as well as blood flow improvement. According to the synergistic actions on γ-GTP and ALP enzymes, it is believed that a higher hair regrowth may be attained by co-administration of LJE and CTE.
It is well known that diverse cytokines and growth factors including EGF and VEGF are involved in the regulation of hair morphogenesis and growth [30,31]. It is of interest to note that dermal papilla cell-derived factors have chemotactic activities on the surrounding cells, which lead, in turn, to promotion of hair growth [32]. EGF is expressed in association with the development of hair follicles [33,34]. Although the expression of both EGF and its receptor (EGFR) in the outer root sheath is down-regulated with the completion of follicular growth, there expression recovers when the follicular cells enter the anagen phase. VEGF plays an important role involved in the angiogenesis during inflammatory reaction and tumor development [35]. Especially, the vascular network is closely associated with the hair growth cycle [36,37,38]. Therefore, it was confirmed that the gene expression of VEGF vary according to the human hair cycle [39].
From the results in the present study, it is explained that the hair growth-promoting effect of Minoxidil may be due to its potentials up-regulating EGF and VEGF that facilitate the follicle growth as well as angiogenesis. Although LJE did not affect VEGF expression, it increased EGF content in the skin after oral administration. By comparison, CTE was effective in the expression of both EGF and VEGF. Notably, LJE and CTE exerted additive effects on the expression of the growth factors.
In addition to the elongation of hairs, the integrity of hair follicles were remarkably improved following treatment with Minoxidil, LJE and/or CTE in the microscopic examination. In early phase of hair loss and baldness in human patients and animal models, the integrity of follicles was impaired and the thickness of hair shaft was reduced, whereas the follicular integrity and shaft thickness recovered in regenerating hairs. Interestingly, such positive effects on the integrity and regrowth of hair components were achieved with oral administration of LJE plus CTE, in which the effect was comparable to that of topical application of a high concentration of Minoxidil. Therefore, it is expected that the combination for oral intake of LJE plus CTE could be a prescription relatively convenient compared to the dermal use of Minoxidil.
In mouse alopecia model, steroid-sensitive lymphocyte infiltration around the hair follicles is common, exhibiting a pattern similar to the immunotherapy of human alopecia with lacrimal gland inflammation [17,40]. In the present study, carrageenan-induced skin inflammation was markedly attenuated by LJE and CTE treatment; i.e., the vascular exudation and inflammatory cell infiltration were synergistically suppressed. In quantitative analysis, major inflammatory mediators NO and PGE2 were substantially controlled following oral co-administration of LJE and CTE. Interestingly, since LJE displayed anti-inflammatory activity higher than that of semi-purified fucoidan [16], it is assumed that there may be additional active ingredient(s), besides fucoidan, in the LJE.
Taken together, the results indicate that combinational oral treatment with LJE and CTE improves hair loss by up-regulating growth factors and related enzyme activities and in part via anti-inflammatory potential. Therefore, it is suggested that a combination including LJE and CTE in appropriate clinical doses and ratios could be a candidate recipe for hair loss patients.
Acknowledgments
This research was financially supported by the Leading Industry Development for Economic Region of the Chungcheong Leading Industry Office, Korea Institute for Advancement of Technology (KIAT) and Ministry of Knowledge Economy (MKE), and in part by High Value-added Food Technology Development Program, Ministry of Agriculture, Food and Rural Affairs (MAFRA; grant number 113034-3).
References
1. Kim JS, Kang BS, Park HJ, Kim DY, Hwang SY, Nam SY, Yun YW, Lee BJ. Promoting effect of herbal extracts mixtures on hair regrowth in C3H/HeJ mice. J Biomed Res. 2009; 10(3):99–108.
2. Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999; 341(7):491–497. PMID: 10441606.

3. Cotsarelis G. The hair follicle: dying for attention. Am J Pathol. 1997; 151(6):1505–1509. PMID: 9403699.
4. Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990; 61(7):1329–1337. PMID: 2364430.

5. Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 2001; 104(2):233–245. PMID: 11207364.

6. Burton JL, Marshall A. Hypertrichosis due to minoxidil. Br J Dermatol. 1979; 101(5):593–595. PMID: 518830.

7. Meisheri KD, Cipkus LA, Taylor CJ. Mechanism of action of minoxidil sulfate-induced vasodilation: a role for increased K+ permeability. J Pharmacol Exp Ther. 1988; 245(3):751–760. PMID: 3385640.
8. Buhl AE, Waldon DJ, Baker CA, Johnson GA. Minoxidil sulfate is the active metabolite that stimulates hair follicles. J Invest Dermatol. 1990; 95(5):553–557. PMID: 2230218.

9. Kaufman KD, Dawber RP. Finasteride, a Type 2 5alpha-reductase inhibitor, in the treatment of men with androgenetic alopecia. Expert Opin Investig Drugs. 1999; 8(4):403–415.
10. Feldman SC, Reynaldi S, Stortz CA, Cerezo AS, Damont EB. Antiviral properties of fucoidan fractions from Leathesia difformis. Phytomedicine. 1999; 6(5):335–340. PMID: 11962540.

11. Wang J, Zhang Q, Zhang Z, Song H, Li P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int J Biol Macromol. 2010; 46(1):6–12. PMID: 19883681.

12. Bojakowski K, Abramczyk P, Bojakowska M, Zwoliñska A, Przybylski J, Gaciong Z. Fucoidan improves the renal blood flow in the early stage of renal ischemia/reperfusion injury in the rat. J Physiol Pharmacol. 2001; 52(1):137–143. PMID: 11321507.
13. Li N, Zhang Q, Song J. Toxicological evaluation of fucoidan extracted from Laminaria japonica in Wistar rats. Food Chem Toxicol. 2005; 43(3):421–426. PMID: 15680677.

14. Shimoda H, Tanaka J, Takahara Y, Takemoto K, Shan SJ, Su MH. The hypocholesterolemic effects of Cistanche tubulosa extract, a Chinese traditional crude medicine, in mice. Am J Chin Med. 2009; 37(6):1125–1138. PMID: 19938221.
15. Yamada P, Iijima R, Han J, Shigemori H, Yokota S, Isoda H. Inhibitory effect of acteoside isolated from Cistanche tubulosa on chemical mediator release and inflammatory cytokine production by RBL-2H3 and KU812 cells. Planta Med. 2010; 76(14):1512–1518. PMID: 20354949.
16. Kyung J, Kim D, Park D, Yang YH, Choi EK, Lee SP, Kim TS, Lee YB, Kim YB. Synergistic anti-inflammatory effects of Laminaria japonica fucoidan and Cistanche tubulosa extract. Lab Anim Res. 2012; 28(2):91–97. PMID: 22787482.
17. Chee E, Fong KS, Al Jajeh I, Nassiri N, Rootman J. The association of lacrimal gland inflammation with alopecia areata. Orbit. 2015; 34(1):45–50. PMID: 25280049.

18. Brzezinska-Wcislo L, Bergler-Czop B, Wcislo-Dziadecka D, Lis-Swiety A. New aspects of the treatment of alopecia areata. Postepy Dermatol Alergol. 2014; 31(4):262–265. PMID: 25254012.
19. Hattori M, Ogawa H. Biochemical analysis of hair growth from the aspects of aging and enzyme activities. J Dermatol. 1983; 10(1):45–54. PMID: 6134761.

20. Lee SH, Lee HJ, Baek IJ, Yon JM, Nam SY, Lee BJ, Yun YW, Kim YB, Hwang SY, Hong JT. Effect of DREAMMO® scalp lotion on hair growth promotion in an alopecia model of C57BL/6 mice. Lab Anim Res. 2006; 22(1):91–96.
21. Handjiski BK, Eichmüller S, Hofmann U, Czarnetzki BM, Paus R. Alkaline phosphatase activity and localization during the murine hair cycle. Br J Dermatol. 1994; 131(3):303–310. PMID: 7918003.

22. Cross MJ, Dixelius J, Matsumoto T, Claesson-Welsh L. VEGF-receptor signal transduction. Trends Biochem Sci. 2003; 28(9):488–494. PMID: 13678960.

23. Shin S, Jeon JH, Park D, Jang JY, Joo SS, Hwang BY, Choe SY, Kim YB. Anti-inflammatory effects of an ethanol extract of Angelica gigas in a Carrageenan-air pouch inflammation model. Exp Anim. 2009; 58(4):431–436. PMID: 19654443.

24. Kim D, Park D, Kyung J, Yang YH, Choi EK, Lee YB, Kim HK, Hwang BY, Kim YB. Anti-inflammatory effects of Houttuynia cordata supercritical extract in carrageenan-air pouch inflammation model. Lab Anim Res. 2012; 28(2):137–140. PMID: 22787488.
25. Rebora A. Pathogenesis of androgenetic alopecia. J Am Acad Dermatol. 2004; 50(5):777–779. PMID: 15097964.

26. Shirai A, Ikeda J, Kawashima S, Tamaoki T, Kamiya T. KF19418, a new compound for hair growth promotion in vitro and in vivo mouse models. J Dermatol Sci. 2001; 25(3):213–218. PMID: 11240269.

27. Kawabe TT, Kubicek MF, Johnson GA, Buhl AE. Use of gammaglutamyl transpeptidase activity as a marker of hair cycle and anagen induction in mouse hair follicles. J Invest Dermatol. 1994; 103(1):122–126. PMID: 7913117.
28. Hamada K, Suzuki K. Evaluation of biochemical indices as a hair cycle marker in C3H mice. Exp Anim. 1996; 45(3):251–256. PMID: 8840142.

29. Park SJ, Lee KW, Lim DS, Lee S. The sulfated polysaccharide fucoidan stimulates osteogenic differentiation of human adipose-derived stem cells. Stem Cells Dev. 2012; 21(12):2204–2211. PMID: 22050637.

30. Danilenko DM, Ring BD, Pierce GF. Growth factors and cytokines in hair follicle development and cycling: recent insights from animal models and the potentials for clinical therapy. Mol Med Today. 1996; 2(11):460–467. PMID: 8947911.

31. Ozeki M, Tabata Y. In vivo promoted growth of mice hair follicles by the controlled release of growth factors. Biomaterials. 2003; 24(13):2387–2394. PMID: 12699676.

32. Fujie T, Katoh S, Oura H, Urano Y, Arase S. The chemotactic effect of a dermal papilla cell-derived factor on outer root sheath cells. J Dermatol Sci. 2001; 25(3):206–212. PMID: 11240268.

33. Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001; 81(1):449–494. PMID: 11152763.

34. Chapman RE, Hardy MH. Effects of intradermally injected and topically applied mouse epidermal growth factor on wool growth, skin and wool follicles of merino sheep. Aust J Biol Sci. 1988; 41(2):261–268. PMID: 3270313.

35. Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995; 146(5):1029–1039. PMID: 7538264.
36. Ellis RA, Moretti G. Vascular patterns associated with categen hair follicles in the human scalp. Ann N Y Acad Sci. 1959; 83:448–457. PMID: 13820052.
37. Stenn KS, Fernandez LA, Tirrell SJ. The angiogenic properties of the rat vibrissa hair follicle associate with the bulb. J Invest Dermatol. 1988; 90(3):409–411. PMID: 2450148.

38. Yano K, Brown LF, Detmar M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J Clin Invest. 2001; 107(4):409–417. PMID: 11181640.

39. Lachgar S, Charveron M, Ceruti I. Distribution of VEGF mRNA indifferent hair follicle stages by fluorescence in situ hybridization combined with confocal laser microscopy. In : Van Neste DJJ, editor. Hair Research for the Next Millennium. Amsterdam: Elsevier;1996. p. 407–412.
40. Freyschmidt-Paul P, Sundberg JP, Happle R, McElwee KJ, Metz S, Boggess D, Hoffmann R. Successful treatment of alopecia areata-like hair loss with the contact sensitizer squaric acid dibutylester (SADBE) in C3H/HeJ mice. J Invest Dermatol. 1999; 113(1):61–68. PMID: 10417620.

Figure 1
Time-course of hair regrowth of mice treated with Minoxidil, or Laminaria japonica extract (LJE) and/or Cistanche tubulosa extract (CTE). ○, normal; •, Minoxidil (3%) application; ▵, 18 mg/kg LJE; ▾, 54 mg/kg LJE; □, 54 mg/kg CTE; ▪, 162 mg/kg CTE; ◇, 18 mg/kg LJE + 54 mg/kg CTE; ♦, 54 mg/kg LJE + 162 mg/kg CTE.
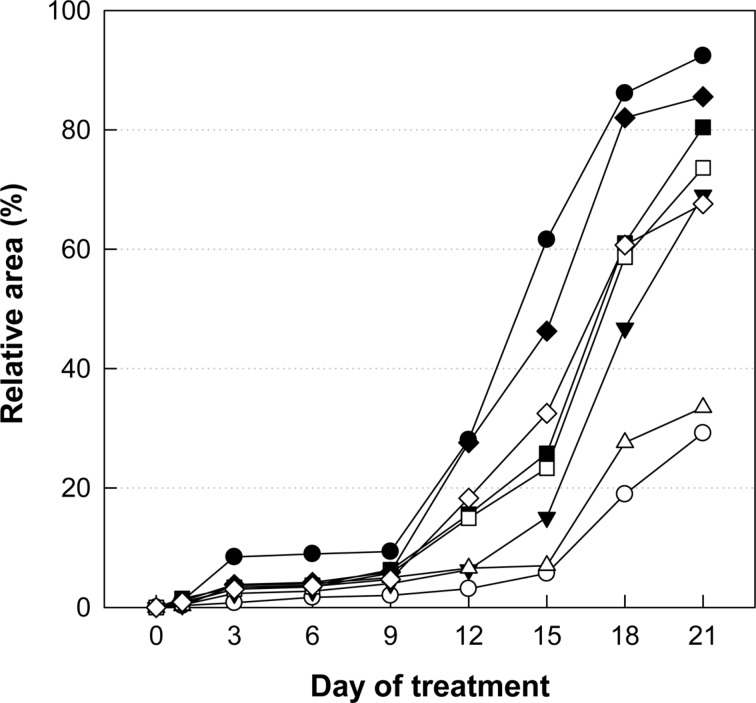
Figure 2
Representative gross findings of hair regrowth of mice treated with Minoxidil, or Laminaria japonica extract (LJE) and/or Cistanche tubulosa extract (CTE) on day 21. A, normal; B, Minoxidil (3%) application; C, 18 mg/kg LJE; D, 54 mg/kg LJE; E, 54 mg/kg CTE; F, 162 mg/kg CTE; G, 18 mg/kg LJE+54 mg/kg CTE; H, 54 mg/kg LJE+162 mg/kg CTE.
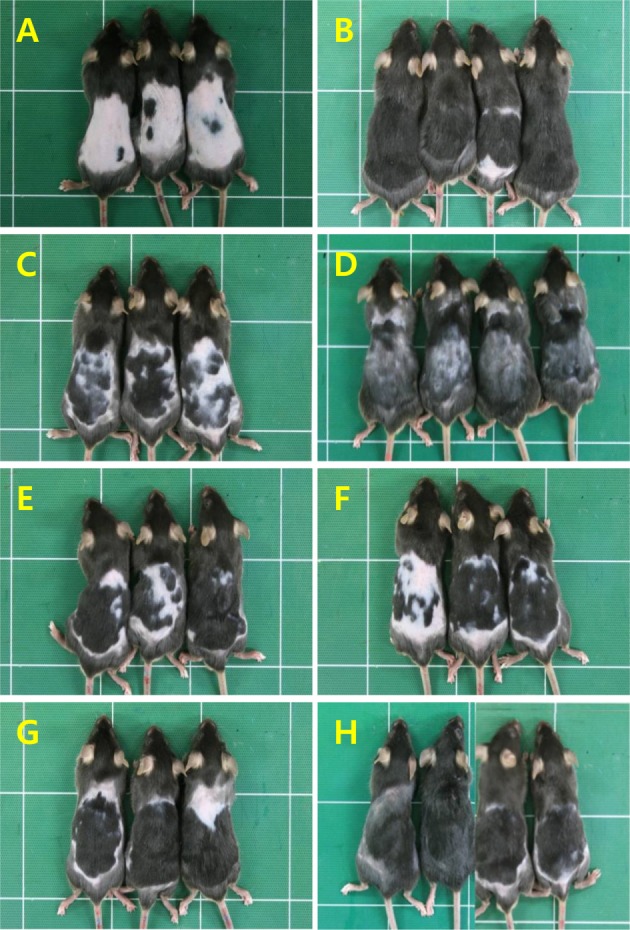
Figure 3
Representative microscopic findings of hair regrowth of mice treated with Minoxidil, or Laminaria japonica extract (LJE) and/or Cistanche tubulosa extract (CTE) on day 21. A, normal; B, Minoxidil (3%) application; C, 18 mg/kg LJE; D, 54 mg/kg LJE; E, 54 mg/kg CTE; F, 162 mg/kg CTE; G, 18 mg/kg LJE+54 mg/kg CTE; H, 54 mg/kg LJE+162 mg/kg CTE.
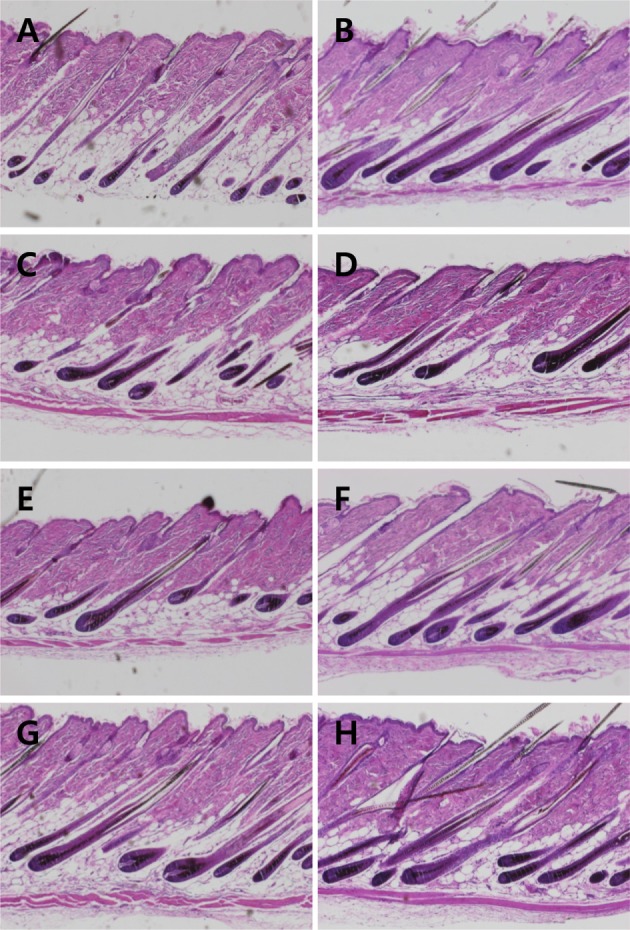
Figure 4
Effects of Minoxidil (MXD, 3%), or Laminaria japonica extract (LJE) and/or Cistanche tubulosa extract (CTE) (in mg/kg) on γ-glutamyl transpeptidase (γ-GTP) activity on days 14 (A) and 21 (B) of treatment. *Significantly different from normal (Nor) control (P<0.05).
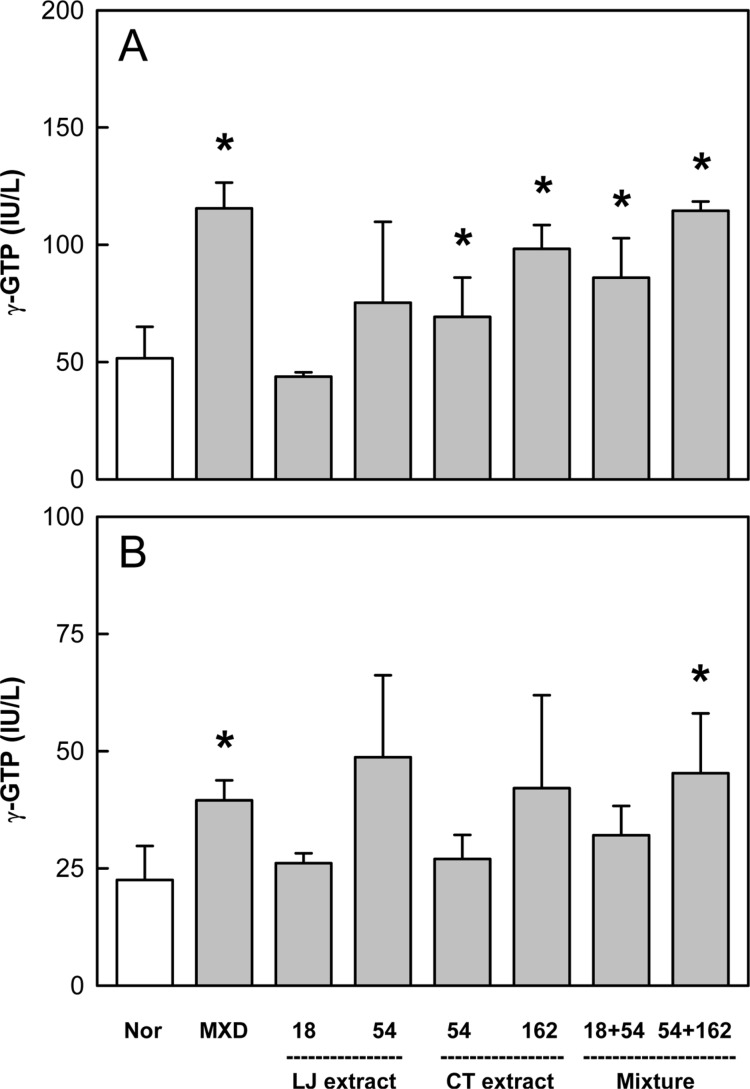
Figure 5
Effects of Minoxidil (MXD, 3%), or Laminaria japonica extract (LJE) and/or Cistanche tubulosa extract (CTE) (in mg/kg) on alkaline phosphatase (ALP) activity on days 14 (A) and 21 (B) of treatment. *Significantly different from normal (Nor) control (P<0.05).
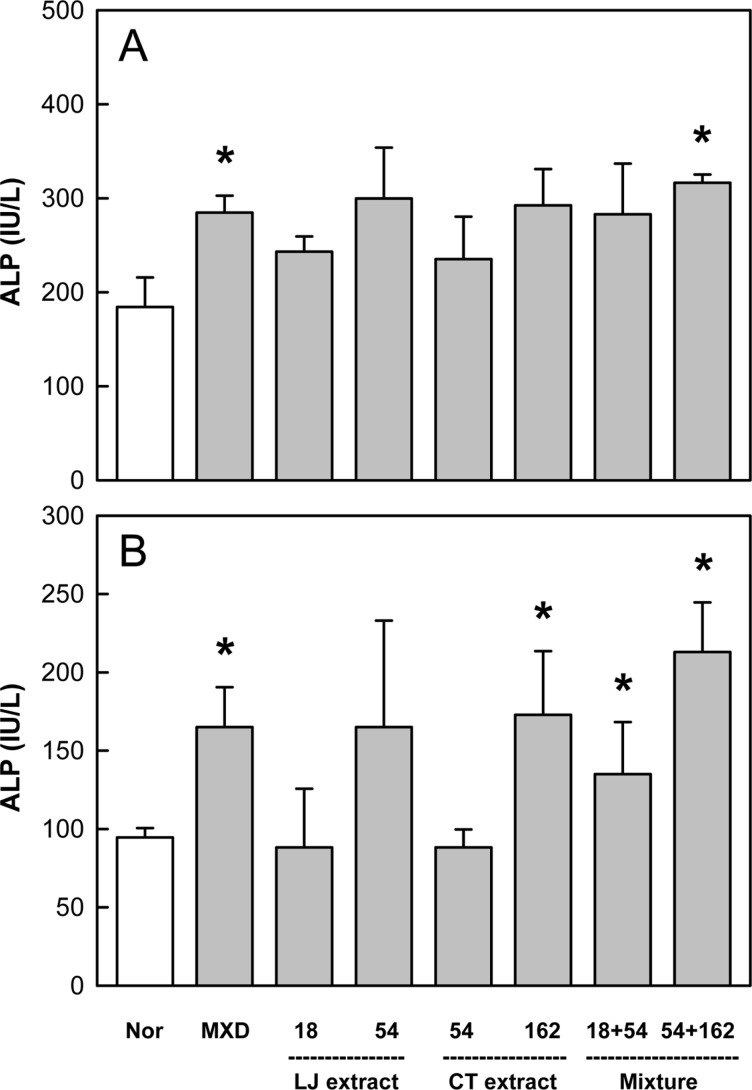
Figure 6
Effects of Minoxidil (MXD, 3%), or Laminaria japonica extract (LJE) and/or Cistanche tubulosa extract (CTE) (in mg/kg) on epidermal growth factor (EGF) content on days 14 (A) and 21 (B) of treatment. *Significantly different from normal (Nor) control (P<0.05).
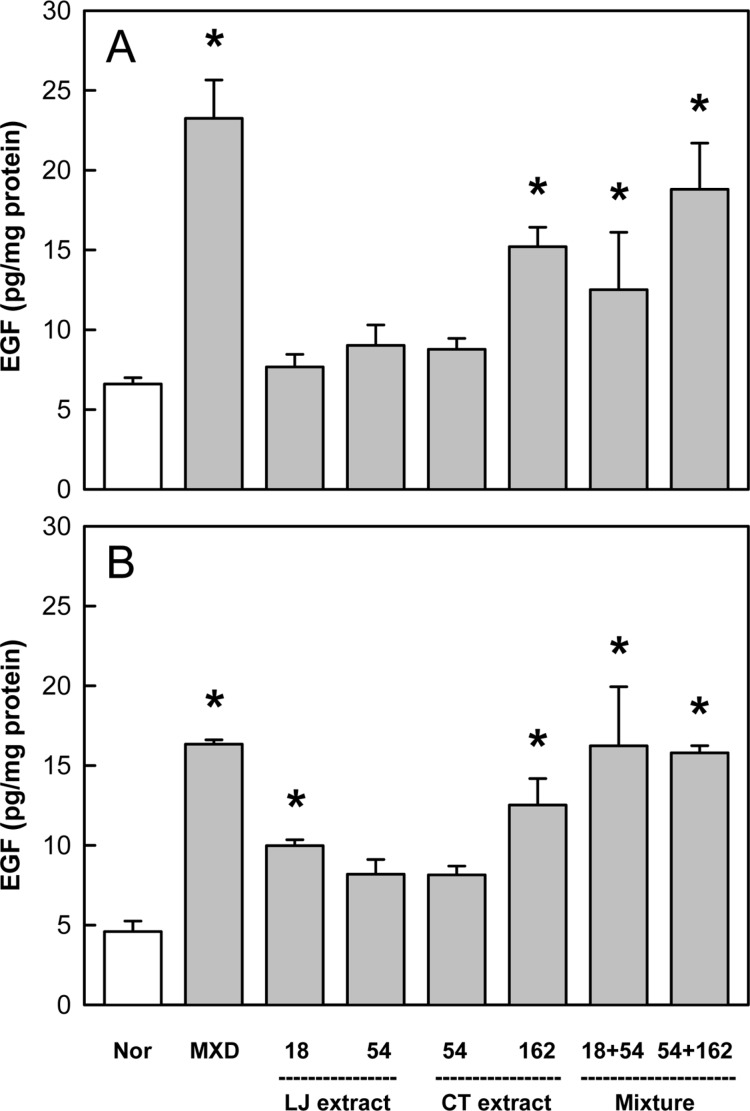
Figure 7
Effects of Minoxidil (MXD, 3%), or Laminaria japonica extract (LJE) and/or Cistanche tubulosa extract (CTE) (in mg/kg) on vascular endothelial growth factor (VEGF) content on days 14 (A) and 21 (B) of treatment. *Significantly different from normal (Nor) control (P<0.05).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download