Abstract
Yerba Mate, derived from the leaves of the tree, Ilex paraguariensis, is widely-used as a tea or as an ingredient in formulated foods. The aim of the present study was to evaluate the effects of Yerba Mate extract on weight loss, obesity-related biochemical parameters, and diabetes in high-fat diet-fed mice.To this end, by using in vivo animal models of dietary-induced obesity, we have made the interesting observations that Yerba Mate has the ability to decrease the differentiation of pre-adipocytes and to reduce the accumulation of lipids in adipocytes, both of which contribute to a lower growth rate of adipose tissue, lower body weight gain, and obesity. Our data from in vivo studies revealed that Yerba Mate treatment affects food intake, resulting in higher energy expenditure, likely as a result of higher basal metabolism in Yerba Mate-treated mice. Furthermore, in vivo effects of Yerba Mate on lipid metabolism included reductions in serum cholesterol, serum triglycerides, and glucose concentrations in mice that were fed a high fat diet. In conclusion, Yerba Mate can potentially be used to treat obesity and diabetes.
Yerba Mate (Ilex paraguariensis), consumed as an herbal tea beverage, is one of the most widely-used plants worldwide. Yerba Mate is commercially packed in individual tea bags or as Mate tea concentrates for use as an ingredient in dietary supplement industries [1-4].
The major difference between green tea and Mate tea production is the drying method. Green tea is dried primarily through fast, high-temperature air drying, but Mate tea is dried very slowly and often using wood smoke. This imparts very different flavor characteristics and contributes to changes in chemical makeup and physical appearance. Numerous active phytochemicals have been identified in Yerba Mate, including polyphenols (chlorogenic acid), xanthines (caffeine and theobromine), purine alkaloids (caffeic acid, 3,4-dicaffeoylquinic acid, and 3,5-dicaffeoylquinic acid), flavonoids (quercetin, kaempferol, and rutin), amino acids, minerals (phosphorous, iron, and calcium), and vitamins (C, B1, and B2) [5-8]. Recently published data have highlighted the beneficial effects of Ilex paraguariensis, which include antioxidant activity [9-11] and inhibition of atherosclerosis [12-14]. In particular, studies have also suggested its potential in the management of obesity [15-17].
Obesity is a growing problem, resulting in significant morbidity and mortality due to weight-related diseases, as well as a reduced quality of life. The defect in energy balance that causes obesity and visceral adiposity is a serious problem that predisposes individuals to complications, such as atherosclerosis, hepatic steatosis, and type 2 diabetes [18]. The increasing incidence of obesity suggests that this epidemic will only worsen in the future [19]. Animal models are useful tools to evaluate the efficacy of potential compounds in the prevention and treatment of obesity. It has been reported that rodents fed a high-fat diet are excellent models of obesity, where the dietary environment is a major contributor [20]. Obesity has become a very important issue in Korean society. Yerba Mate, a potential treatment for obesity, is rarely used in Korea.
The aim of the present study was to evaluate the effects of Yerba Mate extract on weight loss, obesity-related biochemical parameters, and diabetes in high-fat diet-fed mice, as natural products can show different characteristics and pharmacological differences depending on their growing district.
Yerba Mate was collected in Argentina. The collected 1 kg Yerba Mate was thoroughly washed in water, and the sample was extracted 3 times with 10 L distilled water at 100℃ for 2 hours, so extract powder of 200 gram was obtained. The extract was dissolved in purified water prior to oral administration. Yerba Mate extract or vehicle (purified water) was administered by intragastric gavage, in order to guarantee total ingestion. The animals were treated for 4 weeks and received either 0.5, 1, or 2 g/kg instant Yerba Mate/kg body weight.
Male, five-week old C57BL/6J mice (18-23 g), free of specific pathogens, were purchased from SAMTAKO BIO Korea (Osan, Korea). The experiments were performed in accordance with the principles and approval from Ethics Committee of the Wonkwang University, Iksan, Korea (Approval No. WKU11-001). All animals, which were maintained in a temperature-controlled room (temperature 22±2℃, humidity 50±5%) on a 12 h light/dark cycle, were acclimatized to the laboratory environment while housed in individual cages for 1 week before the experiment. Obesity was induced by a 60% fat calorie diet, and mice were randomly divided into 5 groups after the first 6 weeks on the high-fat diet (AIN-93G, cat., #101556; Research Diet, Inc., NJ, USA), as follows: regular diet group (Normal diet), high-fat diet group (HFD, control group), HFD+0.5 g/kg Yerba Mate (HFD+Mate 0.5), HFD+1 g/kg Yerba Mate (HFD+Mate 1.0), and HFD+2 g/kg Yerba Mate (HFD+Mate 2.0). For oral administration, the drugs were diluted in purified water and administered once per day for 4 weeks. At the end of the experiment, mice were deeply anaesthetized (1:1 xylazine:ketamine), and blood samples were collected and stored at -80℃ until they were analyzed.
After 4 weeks of treatment, blood samples were collected and centrifuged at 3,000 rpm for 15 min at 4℃, and serum glucose, triglyceride (TG), total cholesterol (T-CHO), high-density lipoprotein (HDL), low-density lipoprotein (LDL), and leptin levels were measured according to the kit manufacturer's instruction.
Body weight was measured once a week during the feeding period. Internal organs were dissected and weighed. Fat tissue samples also were stored at -80℃ until they were analyzed.
Histological analysis was conducted following a previous reported method [21]. Briefly, the dissected tissues were fixed in 10% neutral buffered formalin for histological analysis and embedded in paraffin. The paraffin-embedded sections were cut at a thickness of 4 µm and stained with hematoxylin and eosin. adipocyte sizes were measured in randomly chosen microscopic areas from independent animals using an Olympus microscope system, and average adipocyte size was calculated by dividing the chosen microscopic area by total adipocyte cell number in the area.
All data were expressed as mean±standard error (SE), and differences between groups were analyzed using one-way ANOVA (Duncan's multiple-range test). Each value was the mean of at least 3 separate experiments in each group, and mean values were considered significantly different when P values were less than 0.05.
We measured the body weights and food intake of the mice once a week after 4 weeks of receiving a Normal diet or HFD with or without Yerba Mate treatment. Compared to HFD mice, Yerba Mate decreased food and water intake in C57BL/6J mice on the HFD (Figure 1). The body weight of the HFD group was increased. Body weight gains were significantly less in Yerba Mate-treated HFD groups. Furthermore, blood glucose was also lowered after treatment with Yerba Mate (Figure 2).
The weights of organs, such as liver, spleen, heart, kidneys, and testes, were increased in HFD mice compared to the HFD group (Figure 3). As shown in Figure 4 and Table 1, the weight of epididymal, retroperitoneal and peri-renal fat tissue in HFD group was increased compared to the normal group, and the fat tissue weight of HFD mice treated with Yerba Mate was much smaller than the HFD group. Additionally, the size of adipocytes from Yerba Mate-treated HFD animals was smaller compared with adipocytes from control HFD animals.
Hepatic steatosis was increased in the HFD group compared to the Normal diet, and it was decreased by Yerba Mate administration, dependent on concentration, but we were unable to observe the hepatocyte balloon and inflammatory cell disposition in the all groups (Figure 5). Histological analysis showed that the adipocyte size changed less in the group receiving Yerba Mate (Figure 6).
To determine if Yerba Mate has an anti-obesity effect in HFD-fed mice, we measured fat weight, TG, T-CHO, HDL-CHO, LDL-CHO, and leptin concentration after 4 weeks during Yerba Mate administration. The Yerba Mate-treated group had significantly lower serum TG and total cholesterol concentrations than did the HFD group. The leptin levels of the Yerba Mate-treated groups were also markedly reduced compared to those of the HFD group (Table 2 and Figure 7).
Mate has central nervous system-stimulant properties that are attributed to its methylxanthine alkaloids, such as caffeine [22], and it is known to contain compounds with antioxidant properties, such as phenolic acids and caffeoylquinic acid derivatives, which are the most abundant compounds in the leaves [23,24]. Other reported effects, including hepatoprotective, choleretic, diuretic, hypocholesterolemic, anti-rheumatic, antithrombotic, anti-inflammatory, anti-obesity, and cardioprotective effects, may partially be explained its popularity [23,25]. Yerba Mate may have benefits over other weight-loss herbal medicines and supplements, the use of which has been clinically linked to adverse events [26].
Because type 2 diabetes is a chronic and progressive disorder, pharmacotherapy will need to address drug-related side effects over longer time periods. Obesity is a well-recognized risk factor for type 2 diabetes, when combined with other known risk factors. It has been an important therapeutic goal to reduce the risk of type 2 diabetes through weight management. To this end, by using in vivo animal models of dietary-induced obesity, we have made the interesting observations that Yerba Mate has the ability to decrease the differentiation of preadipocytes and reduce accumulation of lipids in adipocytes, both of which contribute to lessened growth of adipose tissue, lower body weight gain, and decreased obesity. Our data from in vivo studies revealed that Yerba Mate treatment affects food intake and results in higher energy expenditure, likely from a higher basal metabolism in Yerba Mate-treated mice. Furthermore, in vivo effects of Yerba Mate on lipid metabolism included a significant reduction in serum cholesterol and reduced trends in serum triglyceride and glucose concentrations in mice fed HFD. These factors are the major players in metabolic syndrome and associated disorders.
Yerba Mate has been reported to have various biological activities, which are mainly attributed to its high polyphenol content [24]. Chlorogenic acid, the main polyphenol in Yerba Mate, is thought to modulate the activity of glucose-6-phosphatase, which is involved in glucose metabolism [27], and to reduce the risk of cardiovascular disease by decreasing the oxidation of LDL and cholesterol [28]. In this sense, our results are in accordance with previous studies that have shown that Ilex paraguariensis treatment improves glucose tolerance in obese animals [14,29]. In addition to chlorogenic acid, methylxanthines are also thought to account for some of the pharmacological effects of Yerba Mate [30]. Saponins, another important class of compounds found in Yerba Mate, have been reported to interfere with cholesterol metabolism [31]. Thus, the effects of Yerba Mate on cholesterol levels could be partially attributed to its saponin content. The data presented in this study suggest that Yerba Mate extract may act synergistically to suppress body weight gain and visceral fat accumulation and to decrease the serum levels of cholesterol, triglycerides, and glucose. Adipose tissue is an endocrine organ, which has a fundamental role in metabolism and homeostasis regulation. The production and secretion of an excess or insufficient amount of adipokines greatly influence insulin sensitivity, glucose metabolism, inflammation, and atherosclerosis and may provide a molecular link between increased adiposity and the development of diabetes mellitus, metabolic syndromes, and cardiovascular diseases [32]. In the present study, the level of leptin in serum was directly affected by a high-fat diet. Additionally, treatment with Yerba Mate extract recovered the concentration of leptin.
In conclusion, this study found that Yerba Mate extract has potent anti-obesity activity in vivo. Additionally, we observed that Yerba Mate treatment has a modulatory effect on glucose levels related to obesity.
Figures and Tables
Figure 1
Effects of Yerba Mate on change of food (A) and water (B) intake in a mouse obesity model induced by a high-fat diet. a, b, c, dValues in the row with different superscript letters are significantly different, P<0.05. Data are shown as mean±SE (n=10).

Figure 2
Effects of Yerba Mate on changes in body weight (A) and blood glucose (B) in a mouse obesity model induced by a high-fat diet. a, b, c, dValues in the row with different superscript letters are significantly different, P<0.05. Data are shown as mean±SE (n=10).

Figure 3
Effects of Yerba Mate on organ weight in a mouse obesity induced model by a high-fat diet. a, b, cValues in the row with different superscript letters are significantly different, P<0.05. Data are shown as mean±SE (n=10).

Figure 4
Effects of Yerba Mate on absolute fat weight in a mouse obesity model induced by a high-fat diet. EPI: Epididymal, RP: Retroperitoneal fat, RN: Peri-renal fat. a, b, c, d, eValues in the row with different superscript letters are significantly different, P<0.05. Data are shown as mean±SE (n=10).

Figure 5
Effects of Yerba Mate on histological hepatic steatosis in a mouse obesity model induced by a high-fat diet. A: Normal, B: HFD. C: HFD+Mate 0.5, D: HFD+Mate 1.0, E: HFD+Mate 2.0. (400× magnification). Arrow indicates steatosis (n=10).

Figure 6
Effects of Yerba Mate on histological adipocyte size in a mouse obesity model induced by a high-fat diet. A: Normal, B: HFD. C: HFD+Mate 0.5, D: HFD+Mate 1.0, E: HFD+Mate 2.0, F: quantitative analysis (400× magnification). a, b, c, dValues in the row with different superscript letters are significantly different, P<0.05. Data are shown as mean±SE (n=6).
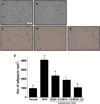
Figure 7
Effects of Yerba Mate on the leptin level in a mouse obesity model induced by a high-fat diet. a, bValues in the row with different superscript letters are significantly different, P<0.05. Data are shown as mean±SE (n=9).

Table 1
Effects of Yerba Mate on percent change in organ weight relative to body weight in an obesity model induced by a high-fat diet

Acknowledgments
This study was supported by a grant of the MICE project of Jeju Island, Ministry of Knowledge Economy, Republic of Korea (70007868).
References
1. Filip R, Lopez P, Giberti G, Coussio J, Ferraro G. Phenolic compounds in seven South American Ilex species. Fitoterapia. 2001. 72(7):774–778.
2. Athayde ML, Coelho GC, Schenkel EP. Caffeine and theobromine in epicuticular wax of Ilex paraguariensis A. St.-Hil. Phytochemistry. 2000. 55(7):853–857.
3. Rosna F, Silvina b, Lotito MS, Graciela F, Ceasar G. Fraga D. Antioxidant activity of Ilex paraguariensis and related species. Nutr Res. 2000. 20(10):1437–1446.
4. Schinella GR, Troiani G, Dávila V, de Buschiazzo PM, Tournier HA. Antioxidant effects of an aqueous extract of Ilex paraguariensis. Biochem Biophys Res Commun. 2000. 269(2):357–360.
5. Michael N, Cillford , Jose R, Ramirez M. Chorogeic acids and purine alkaloids contents of mate (Ilex paraguarinesis) leaf and beverage. Food Chemistry. 1990. 35(1):13–21.
6. Pomilio AB, Trajtemberg S, Vitale AA. High-performance capillary electrophoresis analysis of mate infusions prepared from stems and leaves of Ilex paraguariensis using automated micellar electrokinetic capillary chromatography. Phytochem Anal. 2002. 13(4):235–241.
7. Filip R, Sebastian T, Ferraro G, Anesini C. Effect of Ilex extracts and isolated compounds on peroxidase secretion of rat submandibuulary glands. Food Chem Toxicol. 2007. 45(4):649–655.
8. Zaporozhets OA, Krushynska OA, Lipkovska NA, Barvinchenko VN. A new test method for the evaluation of total antioxidant activity of herbal products. J Agric Food Chem. 2004. 52(1):21–25.
9. Kraemer KH, Taketa AT, Schenkel EP, Gosmann G, Guillaume D. Matesaponin 5, a highly polar saponin from Ilex paraguariensis. Phytochemistry. 1996. 42(4):1119–1122.
10. Gosmann G, Guillaume D, Taketa AT, Schenkel EP. Triterpenoid saponins from Ilex paraguariensis. J Nat Prod. 1995. 58(3):438–441.
11. Miranda DD, Arçari DP, Pedrazzoli J Jr, Carvalho Pde O, Cerutti SM, Bastos DH, Ribeiro ML. Protective effects of mate tea (Ilex paraguariensis) on H2O2-induced DNA damage and DNA repair in mice. Mutagenesis. 2008. 23(4):261–265.
12. Andallu B, Varadacharyulu NCH. Antioxidant role of mulberry (Morus indica L. cv. Anantha) leaves in streptozotocin-diabetic rats. Clin Chim Acta. 2003. 338(1-2):3–10.
13. Chua S, Leibel RL. Obesity genes: molecular and metabolic mechanisms. Diabetes Rev. 1997. 5(1):2–7.
14. Mosimann AL, Wilhelm-Filho D, da Silva EL. Aqueous extract of Ilex paraguariensis attenuates the progression of atherosclerosis in cholesterol-fed rabbits. Biofactors. 2006. 26(1):59–70.
15. Andersen T, Fogh J. Weight loss and delayed gastric emptying following a South American herbal preparation in overweight patients. J Hum Nutr Diet. 2001. 14(3):243–250.
16. Pittler MH, Ernst E. Dietary supplements for body-weight reduction: a systematic review. Am J Clin Nutr. 2004. 79(4):529–536.
17. Opala T, Rzymski P, Pischel I, Wilczak M, Wozniak J. Efficacy of 12 weeks supplementation of a botanical extract-based weight loss formula on body weight, body composition and blood chemistry in healthy, overweight subjects--a randomised double-blind placebo-controlled clinical trial. Eur J Med Res. 2006. 11(8):343–350.
18. Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005. 96(9):939–949.
19. Haslam DW, James WP. Obesity. Lancet. 2005. 366(9492):1197–1209.
20. Bullo M, Casas-Agustench P, Amigo-Correig P, Aranceta J, Salas-Salvado J. Inflammation, obesity and comorbidities: the role of diet. Public Health Nutr. 2007. 10(10A):1164–1172.
21. Lee HA, Hong S, Chung Y, Kim O. Sensitive and specific identification by polymerase chain reaction of Eimeria tenella and Eimeria maxima, important protozoan pathogens in laboratory avian facilities. Lab Anim Res. 2011. 27(3):255–258.
22. Saldana MD, Mohamed RS, Baer MG, Mazzafera P. Extraction of purine alkaloids from maté (Ilex paraguariensis) using supercritical CO(2). J Agric Food Chem. 1999. 47(9):3804–3808.
23. Heck CI, de Mejia EG. Yerba Mate Tea (Ilex paraguariensis): a comprehensive review on chemistry, health implications, and technological considerations. J Food Sci. 2007. 72(9):R138–R151.
24. Bastos DHM, De Oliveira DM, Matsumoto RLT, Carvalho PO, Ribeiro ML. Yerba mate: pharmacological properties, research and biotechnology. Med Aromat Plant Sci Biotechnol. 2007. 1(1):37–46.
25. Bracesco N, Sanchez AG, Contreras V, Menini T, Gugliucci A. Recent advances on Ilex paraguariensis research: minireview. J Ethnopharmacol. 2011. 136(3):378–384.
26. Pittler MH, Schmidt K, Ernst E. Adverse events of herbal food supplements for body weight reduction: systematic review. Obes Rev. 2005. 6(2):93–111.
27. Hemmerle H, Burger HJ, Below P, Schubert G, Rippel R, Schindler PW, Paulus E, Herling AW. Chlorogenic acid and synthetic chlorogenic acid derivatives: novel inhibitors of hepatic glucose-6-phosphate translocase. J Med Chem. 1997. 40(2):137–145.
28. Nardini M, D'Aquino M, Tomassi G, Gentili V, Di Felice M, Scaccini C. Inhibition of human low-density lipoprotein oxidation by caffeic acid and other hydroxycinnamic acid derivatives. Free Radic Biol Med. 1995. 19(5):541–552.
29. Pang J, Choi Y, Park T. Ilex paraguariensis extract ameliorates obesity induced by high-fat diet: potential role of AMPK in the visceral adipose tissue. Arch Biochem Biophys. 2008. 476(2):178–185.
30. Rodriguez de Sotillo DV, Hadley M. Chlorogenic acid modifies plasma and liver concentrations of: cholesterol, triacylglycerol, and minerals in (fa/fa) Zucker rats. J Nutr Biochem. 2002. 13(12):717–726.
31. Han LK, Zheng YN, Xu BJ, Okuda H, Kimura Y. Saponins from platycodi radix ameliorate high fat diet-induced obesity in mice. J Nutr. 2002. 132(8):2241–2245.
32. Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999. 401(6748):73–76.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download