Abstract
Objective
To analyze the factors related to urinary tract infection (UTI) occurrence after an urodynamic study (UDS) in patients with spinal cord injury (SCI).
Methods
We retrospectively investigated the medical records of 387 patients with SCI who underwent UDS with prophylactic antibiotic therapy between January 2012 and December 2012. Among them, 140 patients met the inclusion criteria and were divided into two groups, UTI and non-UTI. We statistically analyzed the following factors between the two groups: age, sex, level of injury, SCI duration, spinal cord independence measure, non-steroidal anti-inflammatory drug use, diabetes mellitus, the American Spinal Injury Association impairment scale (AIS), lower extremity spasticity, a history of UTI within the past 4 weeks prior to the UDS, symptoms and signs of neurogenic bladder, urination methods, symptoms during the UDS and UDS results.
Results
Among the 140 study participants, the UTI group comprised 12 patients and the non-UTI group comprised 128 patients. On univariate analysis, a history of UTI within the past 4 weeks prior to the UDS was significant and previous autonomic dysreflexia before the UDS showed a greater tendency to influence the UTI group. Multivariable logistic regression analysis using these two variables showed that the former variable was significantly associated with UTI and the latter variable was not significantly associated with UTI.
Neurogenic bladder is one of the most common complications in patients with spinal cord injury (SCI) and can cause recurrent urinary tract infection (UTI) and kidney damage during the patient's life span [1]. The state of neurogenic bladder may change during the disease period, and therefore, regular evaluation of bladder function is important. Urodynamic study (UDS) is the most commonly used method for evaluating bladder function [23456]. However, UDS has a few side effects, including UTI [7]. Risk factors for UDS-related UTI have been reported previously, including diabetes, old age (>70 years), history of urination control surgery, indwelling catheterization, in addition to certain procedures such as tissue biopsy [8910], and neurogenic bladder in patients with SCI. In patients with SCI, bladder paralysis, unstable detrusor muscle activity, pyuria before a UDS, and the use of intermittent catheterization or reflex voiding have often been related to UTI occurrence [1112]. Therefore, physicians in many hospitals prescribe prophylactic antibiotics to patients with SCI to prevent UTI when they are going to perform a UDS.
One study reported that UTI can occur after a UDS, even after a course of prophylactic antibiotic therapy [13]. However, the reason for the occurrence of UTI is still unclear. The aim of this study was to determine the risk factors related to UTI occurrence after a UDS during the course of antibiotic prophylaxis in patients with SCI.
We retrospectively reviewed the medical records of patients with SCI who were hospitalized at National Rehabilitation Hospital, in whom the absence of UTI was confirmed, and who underwent UDS between January 2012 and December 2012. A total of 387 patients' records were reviewed, and the exclusion criteria were (1) the patients who did not complete UDS, (2) the patients who were taking antibiotics before UDS, and (3) the patients who were unavailable for observation of the clinical progress for the week after the UDS. Ultimately, 140 patients were included in this study.
In this study, UTI was defined as an infection that met all of the criteria: (1) clinical symptoms that are suspicious for UTI (fever, increased urinary incontinence, lower abdominal pain, increased spasticity, and frequent urination), (2) pyuria (white blood cells >10/HPF) in the urinalysis, (3) bacteriuria (>100,000/mL) in the bacterial culture test [5].
Before undergoing UDS, post-void residual volume (PVR) was measured using a BladderScan BVI 3000 (Verathon, Bothell, WA, USA). After the patient voided or was catheterized, experienced nurses measured PVR multiple times. If maximum volume was more than 100 mL, the patient was categorized as having "presence of residual urine".
In addition, before each UDS in patients with SCI, the presence of UTI was confirmed as follows: a medical examination was performed for detecting UTI symptoms and signs, and urinalysis and a urinary bacterial culture test with antibiotic sensitivity were carried out. After absence of UTI was confirmed, the patients were administered ciprofloxacin 500 mg three times a day for 5 days, starting from the day before the UDS. UDS was performed using the Duet Multi-P urodynamic measuring system (Medtronic, Minneapolis, MN, USA) following the recommendations of the International Continence Society [14]. During observation for 1 week after UDS, only those patients who showed UTI symptoms underwent urinalysis and urine bacterial culture test. Subsequently, according to the definition of UTI, the patients were categorized into one of the two groups, UTI or non-UTI. A comparative analysis was performed for the three domains: general characteristics, items related to neurogenic bladder before UDS, and symptoms during UDS and UDS results.
First, general characteristics included age, gender, level of injury, SCI duration, spinal cord independence measure (SCIM), non-steroidal anti-inflammatory drug (NSAID) use, diabetes mellitus (DM), the American Spinal Injury Association impairment scale (AIS), and lower limb spasticity. Second, the factors related to neurogenic bladder before UDS included a history of UTI within the past 4 weeks prior to the UDS, previous autonomic dysreflexia, urinary incontinence, residual urine, pyuria, bacteriuria and urination method. With respect to a history of UTI, we could not determine the exact result for bacteriuria and antibiotics which were used previously because the patients had been treated at other hospitals. Therefore, we assessed whether the patient had been prescribed antibiotics for UTI in those days. Finally, the presence of autonomic dysreflexia and urinary incontinence during the UDS were compared, and the compared items from the UDS results were as follows: detrusor compliance, maximum detrusor pressure, bladder capacity, bladder sensation, and detrusor activity [15].
Statistical analysis was performed using SPSS ver. 20.0 (IBM, Armonk, NY, USA). Mann-Whitney U test was used to compare the mean value of age, SCI duration and SCIM between the two groups. Chi-square test and Fisher exact test were used to analyze the categorical data for gender, level of injury, NSAID use and DM. Linear by linear association was used for tendency analysis of UTI in relation to AIS and severity of spasticity. Univariate regression analysis was performed to determine the association between UTI occurrence and neurogenic bladder. Using the variables with either statistical or clinical significance, multivariable logistic regression analysis was performed to adjust for confounding factors. The goodness of fit for the final model was confirmed with the Hosmer-Lemeshow test and the predictive power for UTI was indicated by 95% confidence interval (CI) using the C-statistic and the DeLong method. A C-statistic value of 0.7–0.9 was considered to be appropriate, and a p-value less than 0.05 was considered statistically significant.
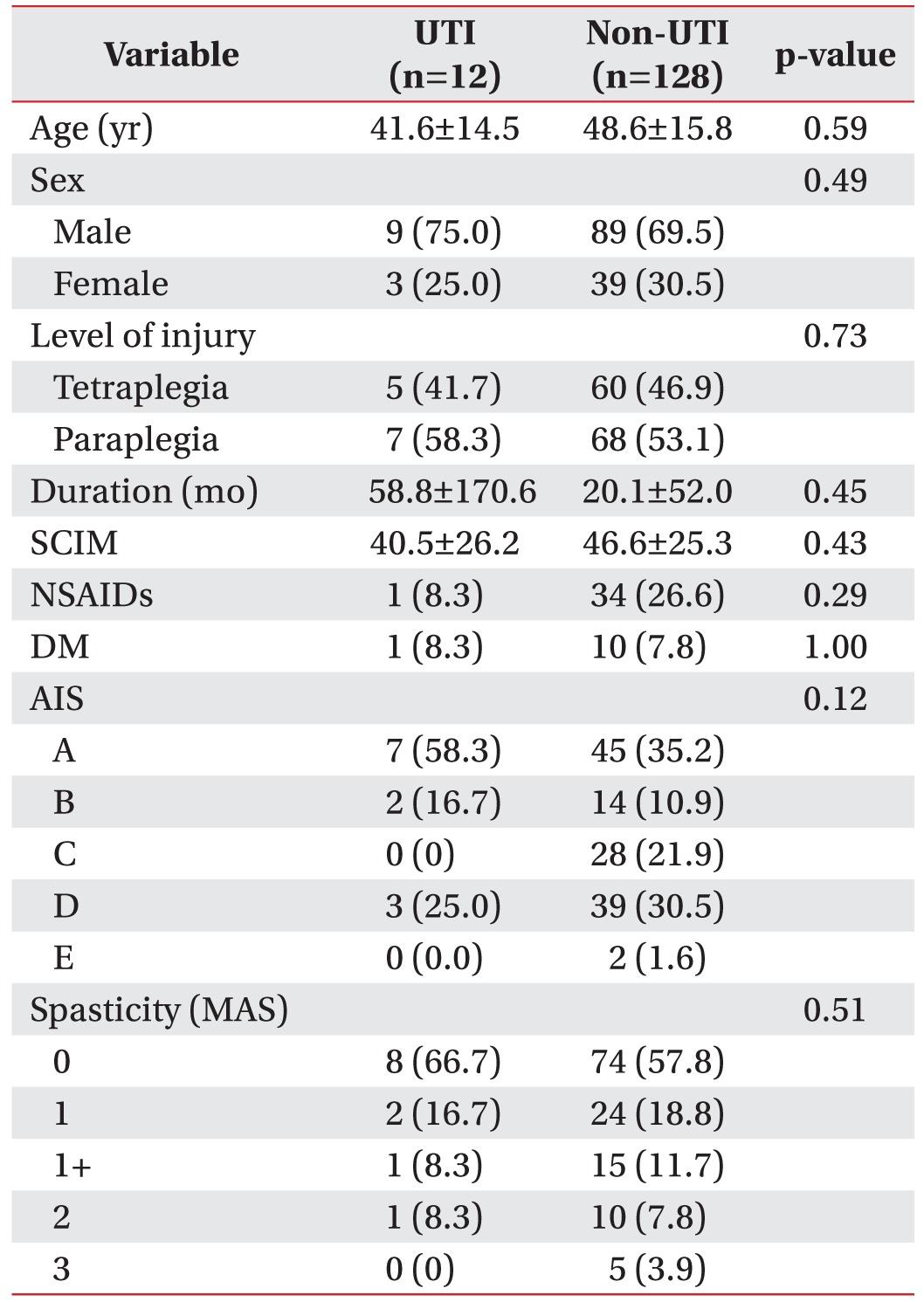
There were 12 participants in the UTI group and 128 participants in the non-UTI group, and the percentage of participants included in the UTI group was 8.6% (95% CI, 4.7%–14.8%).
There were no differences in age, gender, level of injury, SCI duration, SCIM, NSAID use, or presence of DM between the two groups. In addition, there were no statistically significant differences in AIS and the severity of lower limb spasticity between the two groups (Table 1).
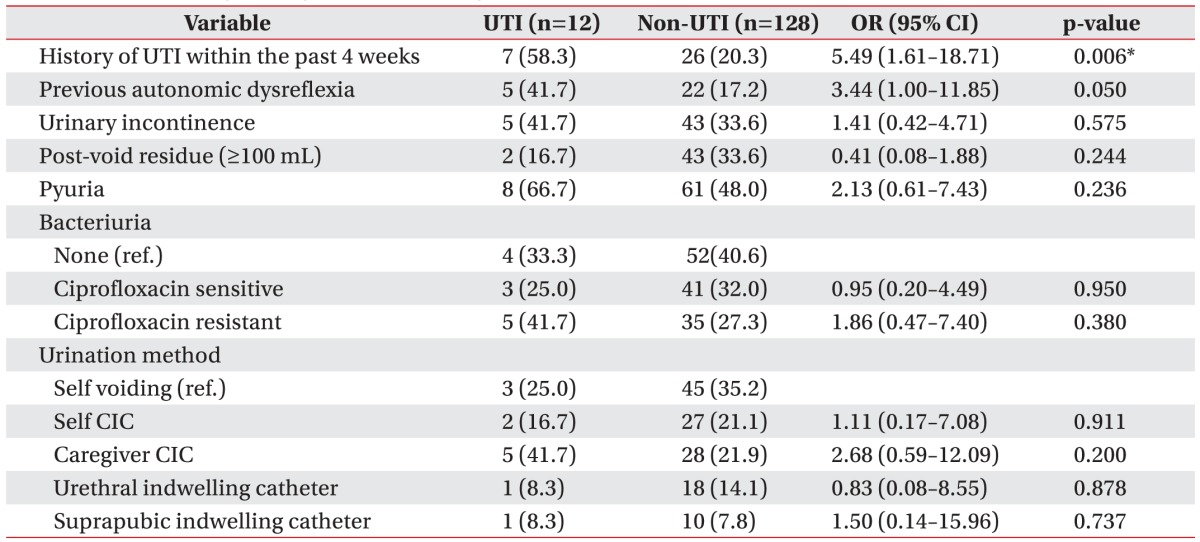
A history of UTI within the past 4 weeks prior to the UDS had a more significant relationship with UTI in the UTI group than in the non-UTI group (p=0.006). Previous autonomic dysreflexia did not show a statistically significant difference between the two groups, but it exhibited a greater tendency to influence the UTI group. There were no significant differences in urinary incontinence, residual urine, or pyuria before UDS between the two groups. In addition, no significant differences were observed in bacteriuria conditions or urination method between the two groups (Table 2).
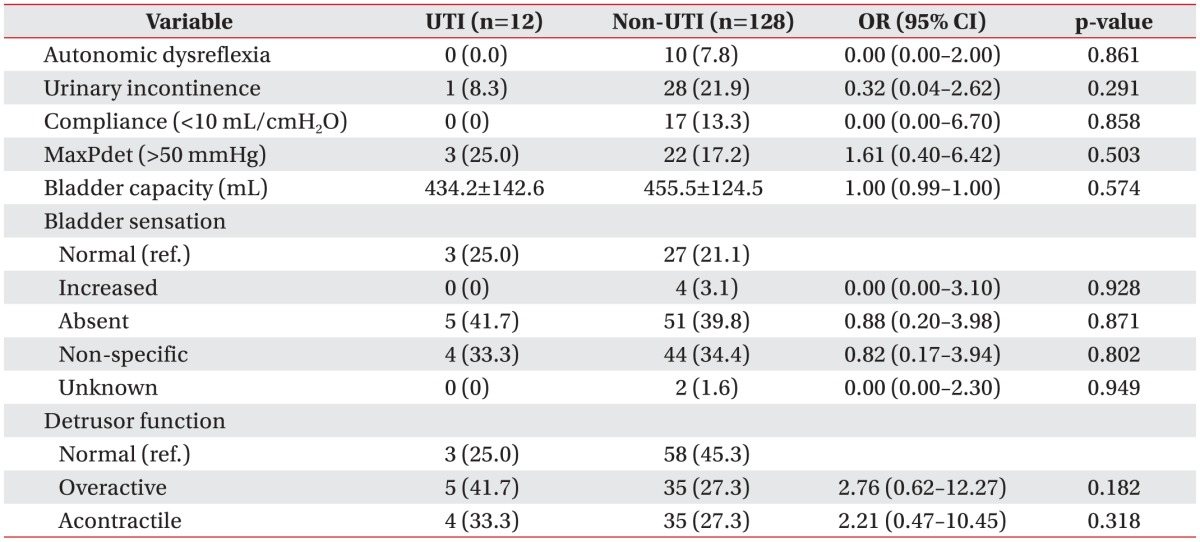
There were no significant differences in the presence of autonomic dysreflexia, urinary incontinence symptom during the UDS, and UDS results between the two groups (Table 3).
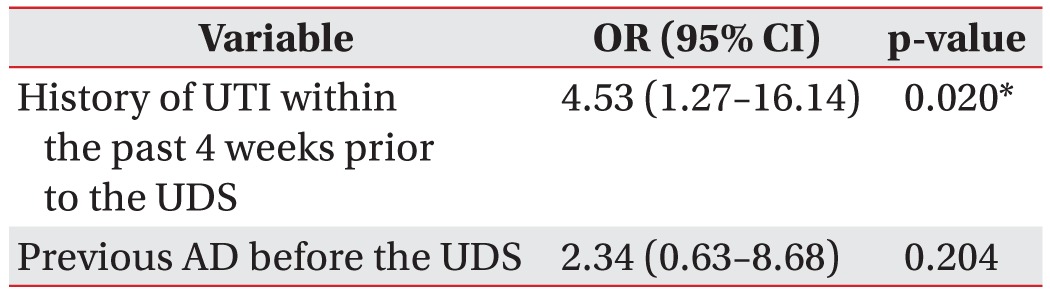
The results of the multivariable logistic regression analysis including a history of UTI within the past 4 weeks prior to the UDS and previous autonomic dysreflexia showed that adjusted risk of UTI occurrence was 4.53 times (95% CI, 1.27–16.14) higher in patients with a history of UTI within the past 4 weeks prior to the UDS, which was statistically significant. Adjusted risk of UTI was 2.34 times (95% CI, 0.63–8.68) higher in patients with previous autonomic dysreflexia, but the difference was not statistically significant (Table 4).
Among the 140 patients with SCI who were scheduled for UDS, the incidence of UTI after UDS was 8.6% despite prophylactic antibiotic therapy. Previous studies have reported that the post-UDS UTI rates ranged from 0% to 20% [111316]. This wide range of rates could be the result of different prophylactic regimens used in the studies.
Patients with SCI who undergo UDS are considered to be at high risk for UTI after UDS, and many studies have reported that prophylactic antibiotic therapy is necessary for patients with SCI before they undergo UDS. Latthe et al. [17] reported that significant bacteriuria decreased by 40% after prophylactic antibiotic therapy, and Foon et al. [13] reported that prophylactic antibiotic therapy reduced the risk of significant bacteriuria compared with that in the placebo group and it also decreased the risk of hematuria. Pannek and Nehiba [18] reported the occurrence of significant UTI in 9.7% of outpatients with demonstrable symptoms and concluded that prophylactic antibiotic therapy should be prescribed to patients with SCI who are about to undergo UDS, since the therapy is relatively safe. There are no standardized criteria for prescribing prophylactic antibiotic therapy for patients who are going to receive a UDS. One study recommended ciprofloxacin as a prophylactic antibiotic; ciprofloxacin is one of the fluoroquinolone drugs, and it is widely applicable to both gram-positive and gram-negative bacteria [11].
However, some researchers have reported that prophylactic antibiotics should be used carefully, considering the occurrence of antibiotic-resistant bacteria and side effects. Darouiche et al. [16] reported that prophylactic antibiotic therapy did not lead to a statistically significant difference in the risk of symptomatic UTI. Naber et al. [9] and Almallah et al. [19] reported that prescribing prophylactic antibiotics before UDS is not necessary unless there is an appropriate indication.
Various factors that are associated with UTI after UDS have been reported. Bothig et al. [12] reported that significant bacteriuria and reflex voiding were risk factors for post-UDS UTI in patients with SCI. The European Association of Urology stated that old age, diabetes, decreased immunity, history of UTI or genitourinary infection, and indwelling catheter are associated risk factors of UTI [20]. Other risk factors for UTI and bacteriuria were reported to be as follows: kidney disease, recurrent UTI, dysuria, artificial heart valves, antibiotic use within a month before the UDS, and pyuria just before UDS [1113192122]. In contrast, UDS findings were found to be not related to the occurrence of UTI after the UDS [81123], and NSAID use has been reported to decrease UTI risk [2425].
The above-mentioned factors were reported in UDS studies that did not assess the use of prophylactic antibiotics or patients without SCI. In this study, we compared the previously reported risk factors of UTI after a UDS with a prophylactic antibiotic. Among these risk factors, only a history of UTI within the past 4 weeks prior to the UDS was found to be statistically significant. A previous study also reported that antibiotic use in the preceding month was a risk factor for UTI after UDS, but the reason was not revealed [11]. Asymptomatic bacteriuria with sensitivity to ciprofloxacin prior to UDS was not significant. We assumed that UTI occurrence after a UDS accompanied by a prophylactic antibiotic was associated with bacteriuria that had caused past symptomatic UTI rather than with asymptomatic bacteriuria prior to the UDS.
There were no significant differences between the two groups with respect to the clinical findings during UDS or the UDS results. These observations were similar to the results from existing studies and UDS findings seemed to have a much lower effect on UTI occurrence.
Our study has several limitations. First, the timing of urinalysis and bacterial culture test before UDS was not standardized. Specifically, urinalysis and bacterial culture test were performed just before UDS in some patients, but other patients were tested at different times. In patients with SCI, asymptomatic pyuria and bacteriuria are common, and intravesical species often vary. Therefore, the susceptibility of bacteria toward specific prophylactic antibiotics can vary. To address this issue, it would be meaningful to perform urinalysis and bacterial culture test just before providing prophylactic antibiotics to all patients who are going to receive a UDS.
Second, we could not confirm the findings for previous bacteriuria based on the antibiotic sensitivity test results or antibiotic use in patients with a history of UTI within the past 4 weeks prior to the UDS because the patients had been treated at other hospitals in those days. If we had been able to include these two data items in our study, the incidence of post-UDS UTI might have been different. This supposition should be confirmed by performing further studies.
Third, there was a considerable difference in the number of participants included in the UTI group and the non-UTI group. In some areas, the number of participants was zero, and thus, there may have been some confusion during the statistical analysis. This was inevitable because of the low incidence of UTI after UDS with prophylactic antibiotic therapy.
Finally, although previous autonomic dysreflexia before UDS was not statistically significant in univariate analysis, the variable was included in the study's multivariable logistic regression model. This was necessary because previous autonomic dysreflexia showed a tendency to influence the UTI group. Moreover, both goodness of fit and the final configured model's predictive power for UTI based on previous autonomic dysreflexia were found to be acceptable (p=0.658, C-statistic=0.7402). Therefore, the multivariable logistic regression model that included previous autonomic dysreflexia was also deemed acceptable.
In conclusion, despite antibiotic prophylaxis, the incidence of post-UDS UTI in patients with SCI was not infrequent in this study. One risk factor of UTI occurrence after UDS with prophylactic antibiotic therapy was a history of UTI within the past four weeks prior to the UDS. We recommend that management prior to a UDS should be carefully performed in patients with SCI who have a history of UTI during that time period.
ACKNOWLEDGMENTS
We thank Bora Lee, a member of the Department of Biostatistic Consulting, for assistance with the statistical analyses.
References
1. Han SJ, Lee JE. Risk factors for urinary tract infection in chronic spinal cord injured patients. J Korean Acad Rehabil Med. 2005; 29:181–186.
2. Cameron AP, Rodriguez GM, Schomer KG. Systematic review of urological followup after spinal cord injury. J Urol. 2012; 187:391–397. PMID: 22177149.

3. Nosseir M, Hinkel A, Pannek J. Clinical usefulness of urodynamic assessment for maintenance of bladder function in patients with spinal cord injury. Neurourol Urodyn. 2007; 26:228–233. PMID: 16998859.

4. Campagnolo DI, Kirshblum S. Spinal cord medicine. 2nd ed. Philadelphia: Lippincott Williams & Wilkins;2011. p. 216–234.
5. Lin VW, Bono CM, Cardenas DD, Frost FS, Hammond MC, Lindblom LB, et al. Spinal cord medicine: principles and practice. 2nd ed. New York: Demos Medical Publishing;2010. p. 263–265.
6. Akkoc Y, Cinar Y, Kismali E. Should complete and incomplete spinal cord injury patients receive the same attention in urodynamic evaluations and ultrasonography examinations of the upper urinary tract? Int J Rehabil Res. 2012; 35:178–180. PMID: 22330305.

7. Onur R, Ozden M, Orhan I, Kalkan A, Semercioz A. Incidence of bacteraemia after urodynamic study. J Hosp Infect. 2004; 57:241–244. PMID: 15236854.

8. Choe JH, Lee JS, Seo JT. Urodynamic studies in women with stress urinary incontinence: significant bacteriuria and risk factors. Neurourol Urodyn. 2007; 26:847–851. PMID: 17580334.

9. Naber KG, Hofstetter AG, Bruhl P, Bichler K, Lebert C;, et al. Guidelines for the perioperative prophylaxis in urological interventions of the urinary and male genital tract. Int J Antimicrob Agents. 2001; 17:321–326. PMID: 11295416.

10. Yenilmez A, Kebapci N, Isikli B, Hamarat M, Donmez T. Morbidity after urodynamic study in diabetic patients. Acta Diabetol. 2009; 46:197–202. PMID: 18989612.

11. Kartal ED, Yenilmez A, Kiremitci A, Meric H, Kale M, Usluer G. Effectiveness of ciprofloxacin prophylaxis in preventing bacteriuria caused by urodynamic study: a blind, randomized study of 192 patients. Urology. 2006; 67:1149–1153. PMID: 16765169.

12. Bothig R, Fiebag K, Thietje R, Faschingbauer M, Hirschfeld S. Morbidity of urinary tract infection after urodynamic examination of hospitalized SCI patients: the impact of bladder management. Spinal Cord. 2013; 51:70–73. PMID: 22964752.

13. Foon R, Toozs-Hobson P, Latthe P. Prophylactic antibiotics to reduce the risk of urinary tract infections after urodynamic studies. Cochrane Database Syst Rev. 2012; 10:CD008224. PMID: 23076941.

14. Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002; 21:167–178. PMID: 11857671.

15. Biering-Sorensen F, Craggs M, Kennelly M, Schick E, Wyndaele JJ. International urodynamic basic spinal cord injury data set. Spinal Cord. 2008; 46:513–516. PMID: 18227849.

16. Darouiche RO, Smith MS, Markowski J. Antibiotic prophylaxis for urodynamic testing in patients with spinal cord injury: a preliminary study. J Hosp Infect. 1994; 28:57–61. PMID: 7806870.

17. Latthe PM, Foon R, Toozs-Hobson P. Prophylactic antibiotics in urodynamics: a systematic review of effectiveness and safety. Neurourol Urodyn. 2008; 27:167–173. PMID: 17849482.

18. Pannek J, Nehiba M. Morbidity of urodynamic testing in patients with spinal cord injury: is antibiotic prophylaxis necessary. Spinal Cord. 2007; 45:771–774. PMID: 17710104.

19. Almallah YZ, Rennie CD, Stone J, Lancashire MJ. Urinary tract infection and patient satisfaction after flexible cystoscopy and urodynamic evaluation. Urology. 2000; 56:37–39. PMID: 10869618.

20. Grabe M, Bjerklund-Johansen TE, Botto H, Wullt B, Cek M, Naber KG, et al. Guidelines on urological infections [Internet]. Bern: European Association of Urology;2011. cited 2015 May 17. Available from: http://uroweb.org/wp-content/uploads/17_Urological-infections_LR-II.pdf.
21. Logadottir Y, Dahlstrand C, Fall M, Knutson T, Peeker R. Invasive urodynamic studies are well tolerated by the patients and associated with a low risk of urinary tract infection. Scand J Urol Nephrol. 2001; 35:459–462. PMID: 11848424.
22. Yokoyama T, Nozaki K, Nose H, Inoue M, Nishiyama Y, Kumon H. Tolerability and morbidity of urodynamic testing: a questionnaire-based study. Urology. 2005; 66:74–76. PMID: 15992874.

23. Athanasiou S, Anstaklis A, Betsi GI, Sotiropoulou M, Falagas ME. Clinical and urodynamic parameters associated with history of urinary tract infections in women. Acta Obstet Gynecol Scand. 2007; 86:1130–1135. PMID: 17712657.
24. Mazumdar K, Dutta NK, Dastidar SG, Motohashi N, Shirataki Y. Diclofenac in the management of E. coli urinary tract infections. In Vivo. 2006; 20:613–619. PMID: 17091768.
25. Akhter T, Baqai R, Aziz M. Antibacterial effect of NSAIDS on clinical isolates of urinary tract infection and diabetic foot infection. Pak J Pharm Sci. 2010; 23:108–113. PMID: 20067876.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download