Abstract
Objective
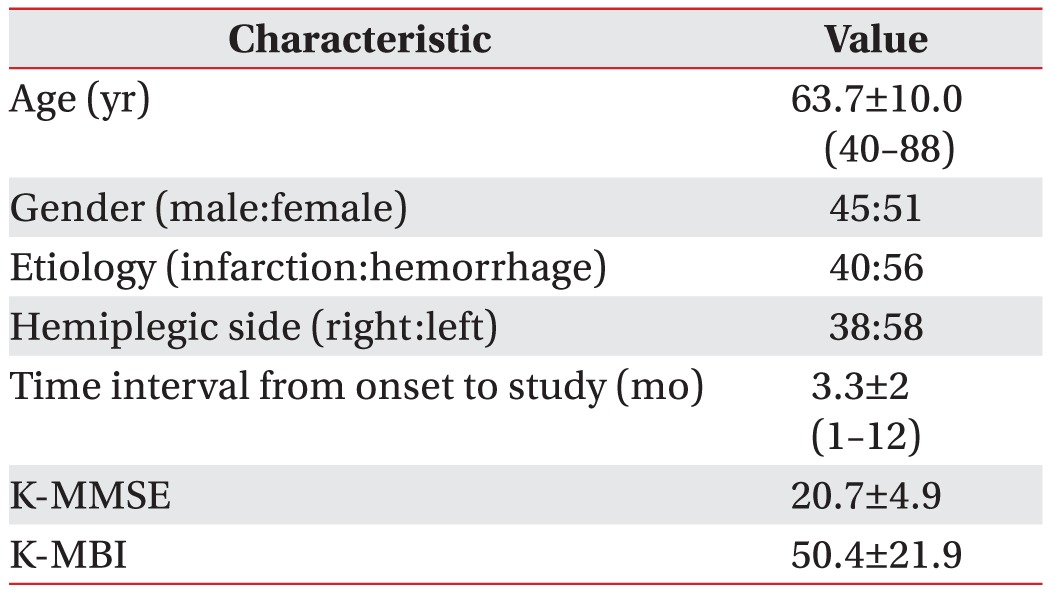
Methods
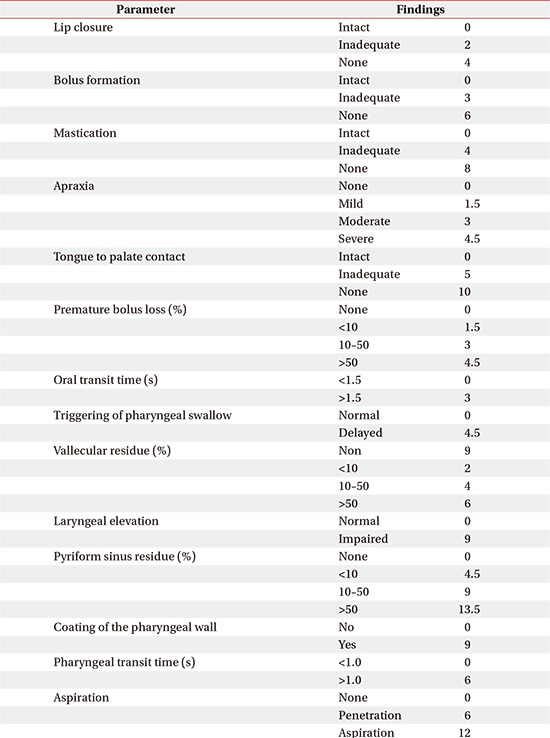
Results
References
Fig. 1
Positioning of the air-filled lingual pressure sensor of the Iowa Oral Performance Instrument between the tongue and an oral structure.
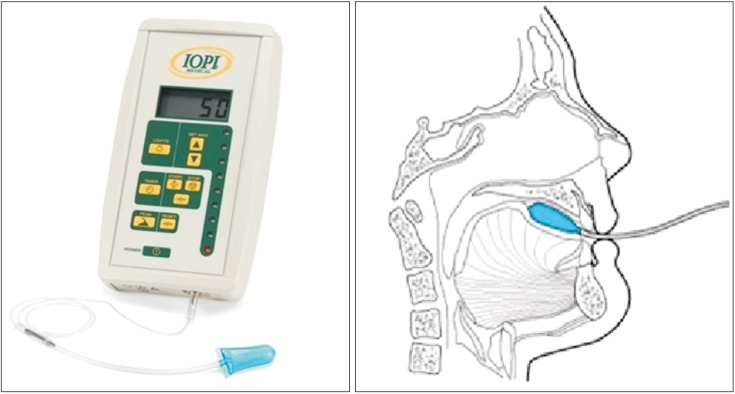
Fig. 2
Correlation between premature bolus loss (PBL) and mean tongue pressure. A larger amount of PBL was associated with a lower maximal tongue pressure (Jonckheere– Terpstra rank correlation test, p<0.05). AP, anterior hard palate-to-tongue pressure; PP, posterior hard palate-to-tongue pressure; BW, buccal-to-tongue pressure on the weak side; BH, buccal-to-tongue pressure on the healthy side; LP, lip closure pressure.
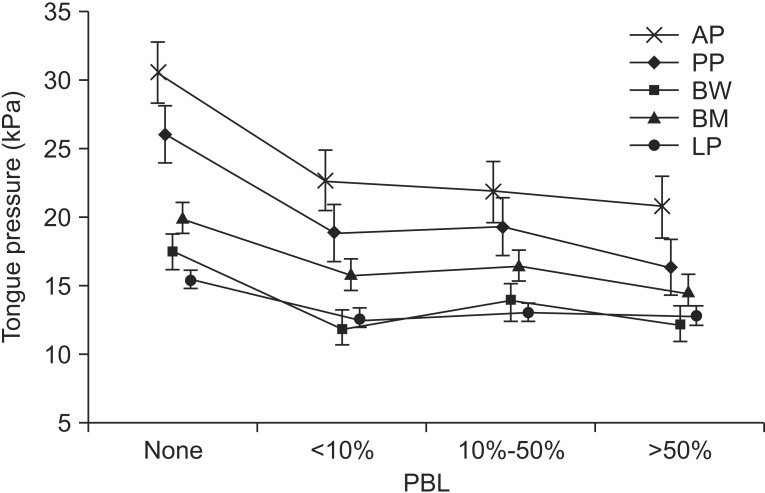
Table 2
The relationships of LP, tongue pressure, and functional outcome with lip closure and bolus formation (unit, kPa)
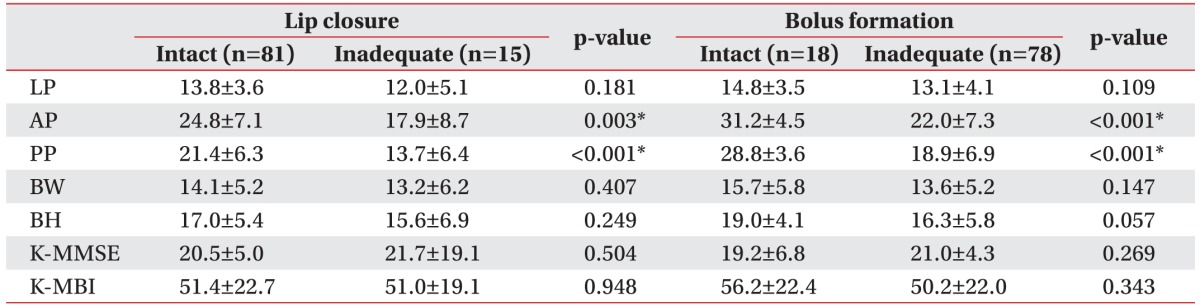
Values are presented as mean±standard deviation.
LP, lip pressure; AP, anterior hard palate-to-tongue pressure; PP, posterior hard palate-to-tongue pressure; BW, buccal-to-tongue pressure on the weak side; BH, buccal-to-tongue pressure on the healthy side; K-MMSE, the Korean version of Mini-Mental State Examination; K-MBI, the Korean version of Modified Barthel Index.
*p<0.05, Mann–Whitney U test.
Table 3
The relationships of LP, tongue pressure, and functional outcome with mastication and oral transit time (unit, kPa)
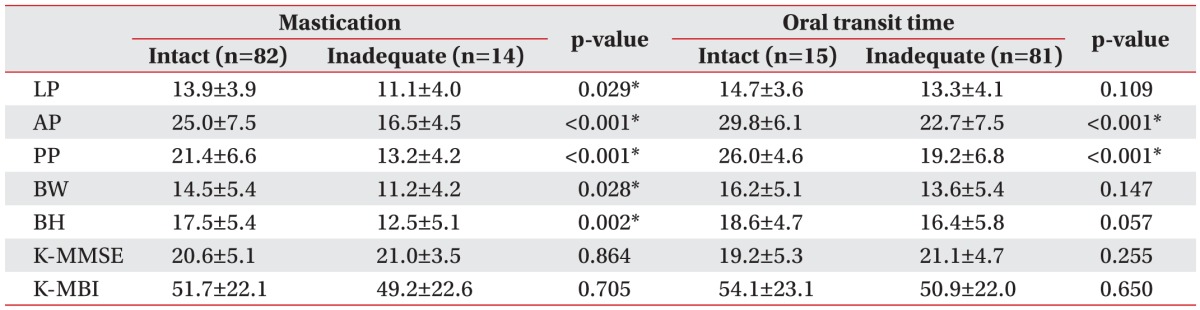
Values are presented as mean±standard deviation.
LP, lip pressure; AP, anterior hard palate-to-tongue pressure; PP, posterior hard palate-to-tongue pressure; BW, buccal-to-tongue pressure on the weak side; BH, buccal-to-tongue pressure on the healthy side; K-MMSE, the Korean version of Mini-Mental State Examination; K-MBI, the Korean version of Modified Barthel Index.
*p<0.05, Mann–Whitney U test.
Table 4
The relationships of LP, tongue pressure, and functional outcome with premature bolus loss and tongue-topalate contact (unit, kPa)
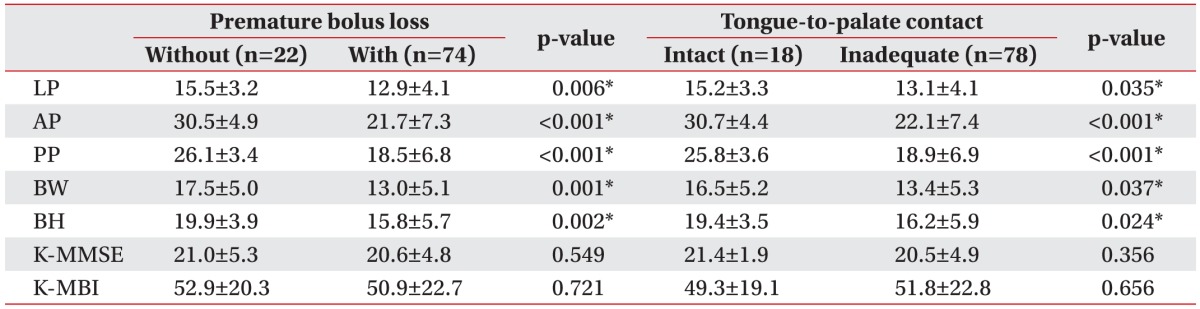
Values are presented as mean±standard deviation.
LP, lip pressure; AP, anterior hard palate-to-tongue pressure; PP, posterior hard palate-to-tongue pressure; BW, buccal-to-tongue pressure on the weak side; BH, buccal-to-tongue pressure on the healthy side; K-MMSE, the Korean version of Mini-Mental State Examination; K-MBI, the Korean version of Modified Barthel Index.
*p<0.05, Mann–Whitney U test.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download