Abstract
Adipose tissue, where various metabolic hormones are secreted, plays a role in metabolizing different substances including androgen. Within fat tissue, enzymes such as aromatase and aldo-keto reductase 1C are responsible for metabolizing testosterone into estrogen and 5-dihydrotestosterone into inactive metabolites. Adipose tissue can also affect the secretion of gonadotropin, which influences the formation of androgen in the testes. At the same time, androgen has an impact on the distribution and proliferation of adipose tissue. The adrenoreceptors for catecholamines, which have been proven to play an essential role in controlling lipolysis, function by being up-regulated by androgens. Furthermore, androgens regulate the activity of lipoprotein lipase, a key enzyme involved in intracellular esterification of adipose tissue.
After the discovery of leptin in the mid-1990s, the role of adipose tissue had begun to gain attention, in that its role could include not only the storage of triglycerides but also the secretion of metabolic proteins.1 Leptin, now known to be released from adipose tissue, regulates the appetite, the balance of energy, and endocrine function, as well as the immune system.2 Other than leptin, adipokines such as adiponectin, resistin, and retinol binding protein-4 are secreted from adipose tissue, affecting insulin resistance.3-5 Furthermore, several studies have suggested that tumor necrosis factor α (TNFα) and interleukin-6, one of the most important cytokines of inflammation, are associated with adipocytes.6,7 In one study, it has been observed that visceral fat tissue promotes systemic inflammation by secreting cytokines into portal circulation.8 This systemic inflammation has an effect on insulin signaling and causes insulin resistance.9
Several receptors and metabolic enzymes for sex hormones mediate metabolism and inactivation of the hormones through their interaction in adipose tissue. Therefore, the concentrations of these circulating sex hormones are influenced by the amount and distribution of the adipose tissue.10 Evidence from a previous study showed that an increased level of testosterone in the bloodstream had caused a rise in insulin sensitivity and a decline in the risk of metabolic syndrome.11 In addition, testosterone was identified to have a negative correlation with obesity, and reciprocally, androgen was found to have an influence on the amount and the distribution of fat.12
This article describes the metabolism of androgen in adipose tissue based on the findings of recent research, and it reviews the effects of androgen on the distribution and metabolism of adipose tissue.
For the past fifty years, it was assumed that the substance responsible for suppression of food intake according to the increase in the stores of energy would exist in the brain.13 This substance had been discovered after homozygous mutation disrupting genes, ob, related to obesity, and db, related to diabetes, were identified in mice.14 An additional study reported that the ob locus had synthesized a substance associated with satiety while the db locus was identified as the crucial gene for this substance to take effect.15,16 This substance is termed leptin, and it was observed that the concentration of leptin increased in the bloodstream as the quantity of body fat increased.17 Another experiment on ob/ob mice with the inability to synthesize leptin and db/db mice with malfunctioning of leptin receptors indicated that both groups of mice showed less movement, less energy expenditure, and increased food intake, leading to obesity and infertility, known to be caused, in this case, by malfunctioning of spermatogenesis in the testes.18,19 When leptin was injected into these mice, only the ob/ob group recovered from infertility.20 These findings imply that obesity is not the sole cause of infertility, but rather, leptin plays a critical role in normal reproduction.
Spermatic cells and Leydig cells in the testes express receptors for leptin.21 This indicates that leptin may play a role in secretion of testosterone and in reproduction. Several lines of evidence have shown that leptin was involved in gonadotropin-stimulated testicular steroidogenesis.22-24 Moreover, an experimental study on mice in vitro suggested that when the anterior pituitary was treated with leptin followed by incubation, the concentrations of luteinizing hormone (LH) and follicle stimulating hormone had increased in proportion to the increase of leptin concentration; it also showed dose-responsiveness with an increase in LH-releasing hormone when the median eminence-arcuate nucleus was treated with low concentrations of 10-12~10-10 M leptin.24 To verify a similar effect of leptin in vivo, leptin was injected into the third ventricle of the ovariectomized estrogen-primed rat; it was found that the LH level increased as expected.24
Leptin also participates in spermatogenesis of the testis. According to research on the distribution of leptin receptors in the testes of the mouse, leptin had an effect on proliferation and differentiation of germ cells through phosphorylation of signal transducer and activator of transcription-3.25 Another study that compared a group of normal mice with a group of leptin-deficient ob/ob mice showed that impaired spermatogenesis, increased germ cell apoptosis, and up-regulated expression of proapoptotic genes were associated with leptin deficiency.26
About 50% of testosterone in adult males is bound to albumin, 44% is bound to sex hormone binding globulin (SHBG), and only 2~3% stays free-form.27 In fact, SHBG is known to decrease the metabolic clearance rate of testosterone and interrupts its movement into cells;28,29 thus, bioavailable testosterone from the tissues is in the unbound as well as the albumin-bound form.
Testosterone can take effect itself or after being transformed by 5α-reductase into the more powerful androgen dihydrotestosterone (DHT). Some of the testosterone is converted to estrogens by aromatase (ARO).30,31 On the other hand, another study discovered that ARO was present in the stromal cells of adipocytes in humans,32 indicating that adipose tissue was involved in the metabolism of sex hormones. Furthermore, increased ARO activity in obese males led to more androgens converting to estrogens, resulting in a higher level of estrogen and a decline of androgen in the plasma.33
It is well established that obesity has an impact on the hypothalamus-pituitary-testicular axis; a study on obese males showed that the Body Mass Index had a negative correlation with the concentration of testosterone and a positive correlation with estradiol.34 The same study indicated that the LH pulse frequency was similar in both normal and obese groups, while the mean diurnal LH levels, mean diurnal LH pulse amplitude, and the sum of all diurnal LH pulse amplitudes and secretory masses were noticeably lower in the obese group.34 Furthermore, an increase in pro-inflammatory cytokines such as TNFα from adipose tissue influences the secretion of gonadotropin in the pituitary.35
DHT, known as a potent androgen, does not get metabolized by ARO, but it has been observed that DHT is converted to the inactive metabolites 5α-androstane-3α and 17β-diol (3α-diol) in fat tissue.36,37 Aldo-keto-reductase 1C (AKR1c), which metabolizes DHT into its inactive form, is expressed in fat tissue.38,39 Evidence from an experiment on human adipose tissue demonstrated that AKR1C mRNA expression and DHT inactivation occurred in subcutaneous and visceral adipose tissue; also, mRNA levels were positively correlated with the amount of visceral adipocytes.37 Furthermore, the androgen inactivation rates were higher in the obese group than the normal group.37 In short, AKR1C mRNA expression increases with the mass of adipose tissue, leading to an increase in androgen inactivation rates.
The receptors for androgen are present in adipose tissue, and are more prominent in visceral (VAT) than subcutaneous adipose tissue (SCAT).40 Among various hormones that regulate the metabolism of adipocytes, catecholamine plays an important role in controlling lipolysis and receptors for catecholamines, known as adrenoreceptors, increase in density by testosterone. Testosterone also influences adipose tissue by up-regulating the adrenoreceptor (AR) that activates lipolysis.41 An experiment on adipose precursor cells of male rats suggested that testosterone stimulated catecholamine-induced lipolysis in a dose-dependent manner through β-AR and adenylate cyclase.42 In particular, lipolysis induced by testosterone was consistently observed, even after treatment with ARO inhibitor, proving that the effect was not due to the estrogens converted by ARO.42 An experiment on male rats in vivo also revealed that androgen had caused a decline in lipolysis through decreasing triglyceride/fatty acid cycling in adipose tissue when the androgen antagonist, cyproterone acetate, was injected into the rats.43 Furthermore, cyproterone acetate reduced catecholamine-induced lipolysis without affecting the activity of esterification enzymes such as GPAT or PPH that were required for the synthesis of triglyceride.43
Lipo-protein lipase (LPL) hydrolyzes circulating triglyceride-rich lipoproteins to facilitate the adipocyte activity of intracellular esterification that utilizes free fatty acids during the process.44 LPL is known to be associated with not only obesity but also with the energy balance, insulin action, and metabolic disorders such as abnormal body weight regulation.45 It is recognized that androgen reduces the LPL activity in adipose tissue, adjusting the central fat accumulation;46 in fact, an experiment that examined the effect of androgen on abdominal adipose tissue suggested that the LPL activity in the tissue had decreased and the triglyceride uptake was inhibited in the group in which testosterone was injected for nine months.47 Such an uptake of triglyceride was more clearly observed in VAT than SCAT, possibly due to the greater number of androgen receptors expressed in VAT.48
Leptin, produced in adipose tissue, influences the functioning of the testes directly and indirectly. The secretion of testosterone is directly mediated by the leptin receptors that are present in the leydig cells of the testes. Moreover, leptin acts on the hypothalamus-pituitary-testicular axis, regulating the functioning of the testes indirectly.
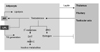
Adipose tissue is involved in the metabolism of androgen to estrogen known to be converted by ARO found in fat tissue. The adipose tissue also affects hypothalamus-pituitary-testicular axis, reducing the release of gonadotropin. A strong androgen DHT, in addition, is converted to an inactive metabolite by AKRIC present in adipose tissue.
Androgens have a negative effect on adipogenesis and lipid synthesis by up-regulating the AR for catecholamine, thus increasing lipolysis. In addition, androgens reduce the LPL activity that is necessary for intracellular esterification of adipocytes, adjusting central fat accumulation.
In conclusion, adipose tissue and androgen influence each other in a bidirectional and reciprocal way (Fig. 1).
Figures and Tables
ACKNOWLEDGEMENTS
H.K. Lee is the main author of this paper; J.K. Lee carried out the literature search; B. Cho is the corresponding author of this paper.
References
1. Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004; 89:2548–2556.

2. Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010; 316:129–139.

3. Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996; 271:10697–10703.

4. Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001; 409:307–312.

5. Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005; 436:356–362.

6. Sopasakis VR, Sandqvist M, Gustafson B, Hammarstedt A, Schmelz M, Yang X, et al. High local concentrations and effects on differentiation implicate interleukin-6 as a paracrine regulator. Obes Res. 2004; 12:454–460.

7. Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001; 280:E745–E751.

8. Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007; 56:1010–1013.

9. Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem. 2003; 278:45777–45784.
10. Rebuffé-Scrive M, Brönnegard M, Nilsson A, Eldh J, Gustafsson JA, Björntorp P. Steroid hormone receptors in human adipose tissues. J Clin Endocrinol Metab. 1990; 71:1215–1219.
11. Muller M, Grobbee DE, den Tonkelaar I, Lamberts SW, van der Schouw YT. Endogenous sex hormones and metabolic syndrome in aging men. J Clin Endocrinol Metab. 2005; 90:2618–2623.

12. Blouin K, Boivin A, Tchernof A. Androgens and body fat distribution. J Steroid Biochem Mol Biol. 2008; 108:272–280.

13. Kennedy GC. The role of depot fat in the hypothalamic control of food intake in the rat. Proc R Soc Lond B Biol Sci. 1953; 140:578–596.
14. Coleman DL. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978; 14:141–148.

15. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994; 372:425–432.

16. Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996; 84:491–495.

17. Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996; 334:292–295.

18. Barash IA, Cheung CC, Weigle DS, Ren H, Kabigting EB, Kuijper JL, et al. Leptin is a metabolic signal to the reproductive system. Endocrinology. 1996; 137:3144–3147.

19. Mounzih K, Lu R, Chehab FF. Leptin treatment rescues the sterility of genetically obese ob/ob males. Endocrinology. 1997; 138:1190–1193.

20. Chehab FF, Lim ME, Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet. 1996; 12:318–320.

21. Banks WA, McLay RN, Kastin AJ, Sarmiento U, Scully S. Passage of leptin across the blood-testis barrier. Am J Physiol. 1999; 276:E1099–E1104.

22. Caprio M, Isidori AM, Carta AR, Moretti C, Dufau ML, Fabbri A. Expression of functional leptin receptors in rodent Leydig cells. Endocrinology. 1999; 140:4939–4947.
23. Tena-Sempere M, Pinilla L, González LC, Diéguez C, Casanueva FF, Aguilar E. Leptin inhibits testosterone secretion from adult rat testis in vitro. J Endocrinol. 1999; 161:211–218.

24. Yu WH, Kimura M, Walczewska A, Karanth S, McCann SM. Role of leptin in hypothalamic-pituitary function. Proc Natl Acad Sci U S A. 1997; 94:1023–1028.

25. El-Hefnawy T, Ioffe S, Dym M. Expression of the leptin receptor during germ cell development in the mouse testis. Endocrinology. 2000; 141:2624–2630.

26. Bhat GK, Sea TL, Olatinwo MO, Simorangkir D, Ford GD, Ford BD, et al. Influence of a leptin deficiency on testicular morphology, germ cell apoptosis, and expression levels of apoptosis-related genes in the mouse. J Androl. 2006; 27:302–310.

27. Dunn JF, Nisula BC, Rodbard D. Transport of steroid hormones: binding of 21 endogenous steroids to both testosterone-binding globulin and corticosteroid-binding globulin in human plasma. J Clin Endocrinol Metab. 1981; 53:58–68.

28. Vermeulen A, Andó S. Metabolic clearance rate and interconversion of androgens and the influence of the free androgen fraction. J Clin Endocrinol Metab. 1979; 48:320–326.

29. Hammes A, Andreassen TK, Spoelgen R, Raila J, Hubner N, Schulz H, et al. Role of endocytosis in cellular uptake of sex steroids. Cell. 2005; 122:751–762.

30. Russell DW, Wilson JD. Steroid 5 alpha-reductase: two genes/two enzymes. Annu Rev Biochem. 1994; 63:25–61.
31. Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev. 1994; 15:342–355.

32. Cleland WH, Mendelson CR, Simpson ER. Aromatase activity of membrane fractions of human adipose tissue stromal cells and adipocytes. Endocrinology. 1983; 113:2155–2160.

33. Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004; 4:579–591.

34. Vermeulen A, Kaufman JM, Deslypere JP, Thomas G. Attenuated luteinizing hormone (LH) pulse amplitude but normal LH pulse frequency, and its relation to plasma androgens in hypogonadism of obese men. J Clin Endocrinol Metab. 1993; 76:1140–1146.

35. Jones TH. Testosterone deficiency: a risk factor for cardiovascular disease? Trends Endocrinol Metab. 2010; 21:496–503.

36. Blouin K, Richard C, Bélanger C, Dupont P, Daris M, Laberge P, et al. Local androgen inactivation in abdominal visceral adipose tissue. J Clin Endocrinol Metab. 2003; 88:5944–5950.

37. Blouin K, Richard C, Brochu G, Hould FS, Lebel S, Marceau S, et al. Androgen inactivation and steroid-converting enzyme expression in abdominal adipose tissue in men. J Endocrinol. 2006; 191:637–649.

38. Zhang Y, Dufort I, Rheault P, Luu-The V. Characterization of a human 20alpha-hydroxysteroid dehydrogenase. J Mol Endocrinol. 2000; 25:221–228.

39. Dufort I, Labrie F, Luu-The V. Human types 1 and 3 3 alpha-hydroxysteroid dehydrogenases: differential lability and tissue distribution. J Clin Endocrinol Metab. 2001; 86:841–846.
40. Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010; 11:11–18.

41. Bouloumie A, Valet P, Dauzats M, Lafontan M, Saulnier-Blache JS. In vivo upregulation of adipocyte alpha 2-adrenoceptors by androgens is consequence of direct action on fat cells. Am J Physiol. 1994; 267:C926–C931.

42. Xu X, De Pergola G, Björntorp P. The effects of androgens on the regulation of lipolysis in adipose precursor cells. Endocrinology. 1990; 126:1229–1234.

43. Hansson P, Saggerson D, Nilsson-Ehle P. Sex difference in triglyceride/fatty acid substrate cycling of rat adipose tissue: indirect regulation by androgens. Horm Metab Res. 1991; 23:465–468.

44. Eckel RH. Lipoprotein lipase. A multifunctional enzyme relevant to common metabolic diseases. N Engl J Med. 1989; 320:1060–1068.
45. Wang H, Eckel RH. Lipoprotein lipase: from gene to obesity. Am J Physiol Endocrinol Metab. 2009; 297:E271–E288.

46. Ramirez ME, McMurry MP, Wiebke GA, Felten KJ, Ren K, Meikle AW, et al. Evidence for sex steroid inhibition of lipoprotein lipase in men: comparison of abdominal and femoral adipose tissue. Metabolism. 1997; 46:179–185.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download