Abstract
Objective
Glycogen synthase kinase 3β (GSK3β) is a pluripotent protein kinase involved in the development of cancers through regulation of numerous oncogenic molecules. Cyclin D1, an important regulator of G1 to S phase transition in various cells, is one of target proteins that GSK3β regulate. Our objective was to assess the expression of GSK3β and cyclin D1 in cervical neoplasm of different histologic grades and to identify their correlation in cervical carcinogenesis.
Methods
Immunohistochemical analysis of GSK3β and cyclin D1 was performed in a total of 137 patients with 12 normal, 62 cervical intraepithelial neoplasia (CIN) (31 CIN1 and 31 CIN3) and 63 invasive cancers including 56 squamous cell carcinomas and 7 adenocarcinomas.
Results
The expression of GSK3β increased in parallel with the lesion grade, while that of cyclin D1 decreased with severity of the lesion (P<0.001). There was a significant inverse correlation between GSK3β and cyclin D1 expression in overall cervical neoplasia (Φ=-0.413, P<0.001). GSK3β expression was higher in squamous cell carcinoma than in adenocarcinoma (P=0.049).
Conclusion
These results suggest that the expressional increase in GSK3β plays a role in cervical carcinogenesis and has inverse correlation with cyclin D1 expression in this process. In addition, GSK3β expression appears to be associated with the histologic type of cervical cancer, especially squamous cell carcinoma.
Cervical cancer is the fourth most common malignancy worldwide and second most leading malignancy in female, especially in young women between 15 and 45 years of age. Despite the improvement in health policies and dissemination of vaccines, survival rate have not changed in the past years, especially in developing countries, and is still an important cause of death among women [1].
The cervical carcinoma is associated with persistent infection by high-risk human papilloma virus (HPV) infection [2]. HPV-16 and HPV-18 are the most commonly detected high-risk HPV types involved in cervical cancer [1]. Many studies have proved that integration of high-risk HPV DNA into host cell genome results in increased expression of oncoproteins E6 and E7, which interact with tumor suppressor proteins: p53 and retinoblastoma protein (Rb), respectively [345]. These interactions alter the cell cycle control by creating uncontrolled activation and cause a lenient state for non-repaired mutations and chromosomal instability [246]. HPV oncoproteins can also modify many biological pathways like Wnt/β-catenin signaling or mitogen-activated protein inase signaling and alter transcription factor like nuclear factor-kappaB (NF-kB) to maintain malignant processes [78].
Glycogen synthase kinase 3β (GSK3β) is a multifunctional protein kinase involved in numerous cellular processes; cell proliferation, differentiation, and cell cycle regulation [910]. The activity of GSK3β is regulated by site-specific phosphorylation. It is activated by phosphorylation at tyrosine (Tyr216 in GSK3β, pY216GSK3β) and inhibited by phosphorylation at serine (Ser9 in GSK3β, pS9GSK3β) [911]. GSK3β is a key regulator of various oncogenic molecules such as c-Myc [912], p53, c-Jun, AP-1 [9], cAMP response element-binding protein [13], and cyclin D1 [914]. It is also involved in multiple signaling pathways, playing an inhibitory role in Wnt/β-catenin signaling or an activating role in NF-kB mediated pathway, leading to cancer cell survival [1516]. However, its role in cancer remains contradictory because it has shown to have paradoxical roles in various cancers as a tumor suppressor in skin, larynx, and oral cancer [1718] or as a tumor promotor in cancers of colon [19], pancreas [15], bladder [20], and ovary [21], renal cell carcinoma [22] and glioblastoma [23].
Cyclin D1, together with its catalytic subunits known as cyclin dependent kinases (CDK4 and CDK6), is an important regulator of G1 to S phase transition in various cell types [182425]. Overexpression of cyclin D1 has been observed frequently in human cancers and has been proven to play an important role in neoplastic transformation [262728]. In cervical cancer, expression of cyclin D1 is aberrant. Some studies have found elevated levels of this protein [29], whereas others have observed it to be underexpressed [3031]. Cyclin D1 turnover, which is dependent on threonine286 phosphorylation, is catalyzed by GSK3β [14]. The phosphorylation of cyclin D1 leads to its nuclear export and proteasomal degradation in the cytoplasm [14]. Therefore, inverse correlation between GSK3β and cyclin D1 would be assumed.
In the uterine cervix, GSK3β expression and its significance in cervical cancer remain unknown, although there is a report on the expression on the phosphorylated form of GSK3β in cervical cancer [32]. Moreover, there has been no study on the correlation between GSK3β and cyclin D1 in cervical neoplasm. The purpose of this study is to assess the expression of GSK3β and cyclin D1 and their association in different stages of cervical cancer progression. Our data showed increased GSK3β expression in cervical cancer, especially in squamous cell carcinoma (SCC), and decreased expression of cyclin D1 in cervical cancer, suggesting a possible involvement of GSK3β and cyclin D1 in cervical carcinogenesis.
The cervical tissue specimens, collected by LEEP (loop electrosurgical excision procedure), conization or hysterectomy, were retrieved from the files of the Department of Pathology, Eulji General Hospital, Eulji University School of Medicine, Seoul, Korea from 2000 to 2015. A total of 137 patients with 31 cervical intraepithelial neoplasia (CIN) 1, 31 CIN3, 63 invasive cancers (56 SCCs and 7 adenocarcinomas) and 12 normal cervical tissues from the uteri removed for non-cervical pathologic conditions were included in this study. The age of cancer patients ranged from 22 to 81 years and the mean was 47.5 years. The stage of 63 invasive cervical cancer patients was classified into 23 cases of stage Ia, 23 stage Ib, 5 stage II, 10 stage III, and 2 stage IV according to the International Federation of Gynecology and Obstetrics (FIGO) staging system. The HPV data of the patients were not available and the authors used p16 immunostaining as an ancillary test for a marker of HPV infection. After reviewing all hematoxylin and eosin-stained slides from each case, representative paraffin blocks of each lesion were selected and tissue microarray was constructed as previously described [33]. The study was performed with the approval of the institutional review board of Eulji General Hospital.
Immunohistochemical staining was performed using an autostainer (DakoCytomation, Carpinteria, CA, USA). Four-µm-thick tissue sections were obtained from tissue microarray blocks and mounted on poly-L-lysine coated slides. After deparaffinization and rehydration, antigen retrieval was performed by heating the sections in citrate buffer (pH 6.0) at 121℃ for 10 minutes. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 5 minutes, and the sections were incubated with antibodies against GSK3β (BD Biosciences, Lexington, KY, USA; 1:20), cyclin D1 (Dako, Glostrup, Denmark; 1:100) and p16 (p16INK4a kit). Color development and counterstaining of the sections were performed by diaminobenzidine and hematoxylin. GSK3β expression was considered as positive when more than 10% of the tumor area showed cytoplasmic with/without nuclear staining and categorized according to the intensity as weak (+), moderate (++), and strong (+++). Cyclin D1 expression was considered as positive if the percentage of nuclear staining exceeds 10% of the tumor area. The expression of p16 was considered as positive when more than 10% of the tumor area showed both nuclear and cytoplasmic staining.
Comparative analysis of immunoexpression between CIN1, CIN3, and invasive cervical cancer was performed using chi-square test and Fisher’s exact test. The correlation between cyclin D1 and GSK3β expression was assessed using phi coefficient. The association between GSK3β expression and clinicopathologic parameters, such as tumor size, FIGO stage, lymphovascular invasion, lymph node metastasis, and histology were analyzed using chi-square or Fisher’s exact test, respectively. In all of the experiments, a P-value <0.05 was considered statistically significant. Statistical analysis of the data was performed using the SPSS ver. 14.0 (SPSS Inc., Chicago, IL, USA).
The staining pattern of GSK3β and cyclin D1 in precancerous and cancerous cervix tissues is shown in Fig. 1. GSK3β was negative in normal epithelia, but cyclin D1 immunostaining was positive in the basal and parabasal epithelia of all normal cervical specimens. p16 was stained with patchy or diffuse basal pattern in all CIN1 specimens. All CIN3 and SCC specimens showed moderate to strong p16 staining with diffuse basal pattern. The expression of GSK3β increased with progression of disease from CIN1 (3.2%) to CIN3 (54.8%) to cancer (76.2%). Most specimens of CIN1 showed no immunoreactivity of GSK3β. In CIN3, immunostaining for GSK3β was mostly weak and strong immunostaining was observed in cancer tissues only. Cyclin D1, found present in all normal (12/12) and CIN1 specimens (31/31), decreased with disease progression but slight increase was observed in cancer (12.7%) compared to CIN3 (9.7%) (Table 1).
The expressional relationship between GSK3β and cyclin D1 was at inverse correlation in general (φ=-0.413, P<0.001, data not shown). Among 62 CIN1 and CIN3 cases, 18 (29.0%) were GSK3β positive, 15 (83.3%) of which were cyclin D1 negative, while out of 44 (71.0%) GSK3β negative cases, 31 (70.5%) were cyclin D1 positive. This result showed the inverse correlation between GSK3β and cyclin D1 with progression in precancerous lesions (φ=-0.491, P<0.001) (Table 2A). In CIN3 and SCC, however, out of 65 GSK3β positive specimens, 56 were cyclin D1 negative (86.2%) while only 2 out of 29 GSK3β negative specimens were cyclin D1 positive (6.9%). Therefore, there was no significant correlation between GSK3β and cyclin D1 when CIN3 progressed to invasive cancer (φ=0.100, P=0.333) (Table 2B).
The staining of GSK3β was mostly weak in overall CIN but its intensity increased in cancer, showing moderate (18/48, 37.5%) and strong (6/48, 12.5%) staining intensity in half of the cancer specimens (Table 3). With increasing intensity, GSK3β expression was more frequently observed in the nucleus as well as in the cytoplasm.
The expression of GSK3β was also analyzed according to clinicopathological parameters. GSK3β was found to be dependent on histological type (P=0.049). SCC was more related to the GSK3β expression. Out of 56 SCC cases, 45 (80.4%) were GSK3β positive and more than half (23/45, 51.1%) of them showed moderate to strong staining intensity (Fig. 2A). In adenocarcinoma, most cases were GSK3β negative or weakly stained (Fig. 2B). In the evaluation of the association between GSK3β expression and tumor size, FIGO stage, lymphovascular invasion, and lymph node metastasis, overall expression of GSK3β tended to increase with larger tumor size, greater FIGO stage and lymph node metastasis, but all failed to attain statistical significance. The results are summarized in Table 3.
GSK3β is implicated in numerous cellular processes, regulating various transcription factors and proteins such as c-Myc, NF-kB, p53, β-catenin, and cyclin D1, through phosphorylationdependent inactivation or activation [910111234]. GSK3β plays diverse roles in human diseases including diabetes, immunologic and neurologic disorders, and malignancies [91035]. In human cancers, GSK3β acts as a tumor promoter or suppressor, depending on the tumor cell type [17].
In the present study, we found an elevated expression of GSK3β in parallel with the lesion grade. The staining intensity was also significantly increased in invasive cancer compared to CIN3, suggesting the involvement of GSK3β in cervical cancer progression. It has been shown that GSK3β may contribute to cancer cell survival and proliferation by transcriptional activation of NF-κB target genes XIAP and Bcl-2 in bladder and pancreatic cancers [1520]. In glioblastoma cells, GSK3β appears to promote cell survival and proliferation by protecting the tumor cells from apoptosis via the inactivation of p53- and/or Rb-mediated pathways [23]. Whether GSK3β affects NF-κB mediated cell proliferation or the inactivation of p53 and Rb in cervical cancer remains to be investigated.
The previous studies on GSK3β in human cancers showed nuclear accumulation of GSK3β in tumor cells, which is compatible with the role of GSK3β as a key regulator of various nuclear proteins [11152022]. In the present study, distinct subcellular expression pattern of GSK3β could not be determined because nuclear/cytosolic fractionation was not done. However, closely looking into our data, the amount of GSK3β increased immensely in the cytoplasm prior to nuclear accumulation and thereafter GSK3β nuclear staining increased, corresponding with the previous data [152022].
Here, the intensity of GSK3β cytoplasmic and nuclear staining was generally stronger in cervical cancer (24/48, 50%) than in CIN3 (2/17, 11.8%) (Table 3) This might serve as a valuable marker for pathologic progress of cervical cancer. Many other cancers such as bladder cancer and pancreatic cancer have already proved potentials of using GSK3β as a prognostic marker [1522]. These studies came to a conclusion that the nuclear accumulation of GSK3β has strong correlation with the poor prognosis, worse survival and high-grade tumors. Our specimens mostly included precancerous lesions and low-stage cancers because the treatment of choice in high-grade tumors is concurrent chemoradiation therapy. As cervical cancer staging is decided clinically, high-stage tumor patients were mostly excluded. If we were able to obtain specimens of these excluded patients, our results might have consolidated the results of the preceding studies. Additional studies, especially with patients at more advanced stage of cervical cancer, are needed to investigate whether this nuclear accumulation is correlated with the prognosis, survival or high-grade tumors of cervical cancer.
However, we found that GSK3β immunostaining was significantly associated with histologic type of cervical cancer, even though no correlation between other clinicopathologic parameters and GSK3β expression was seen. GSK3β expression had more relevance with SCC, although not enough adenocarcinoma specimens were retrieved. In oral cavity, GSK3β expression is also higher in SCC than in non-SCC, such as mucoepidermoid carcinoma, adenoid cystic carcinoma, and basal cell carcinoma [18]. These findings may indicate the involvement of GSK3β in the squamous differentiation of cancer cells.
In SCC, the study on the expression of GSK3β has been mainly focused on the phosphorylated form of GSK3β (pS9GSK3β and pY216GSK3β). As described above, in oral SCC the expression level of pS9GSK3β is also up-regulated as well as GSK3β increase [18]. In cervical neoplasm, the study on the expression of pS9GSK3β and pY216GSK3β showed overexpression of pS9GSK3β and c-Myc and decreased expression of pY216GSK3β in CIN and SCC [32]. In that study, the expression of pS9GSK3β and c-Myc is positively correlated, suggesting the involvement of pS9GSK3β in the activation of Wnt/β-catenin pathway in cervical carcinogenesis [32]. Taken together with our data, GSK3β seems to increase in proportion to the expression of pS9GSK3β in the progress from CIN and SCC. GSK3β and pS9GSK3β might be implicated in different signaling pathway or cellular mechanism in cervical carcinogenesis. Following studies on the expression and correlation of GSK3β with pS9GSK3β and pY216 GSK3β in cervical cancer would be required.
In the present study, cyclin D1 expression decreased with increase in GSK3β staining intensity. This inverse expression pattern of GSK3β and cyclin D1 might support the role of GSK3β as a regulator of cyclin D1 proteolysis [14]. Nuclear import of GSK3β causes redistribution of cyclin D1 from the cell nucleus to the cytoplasm leading to proteasomal degradation of cyclin D1 [14]. In cervical cancer, data on cyclin D1 expression level are conflicting. Cheung et al. [27] and Nichols et al. [36] reported cyclin D1 amplification and protein overexpression in cervical cancer. In contrast, Bae et al. [31] reported reduced cyclin D1 mRNA and protein expression in cases of CIN and SCC compared to normal cervical tissue. This might be because cyclin D1 is no longer required for G1 progression in cells transformed by HPV due to binding of HPV E7 to the Rb leading to the release of E2F transcription factor [37]. They also showed gradual increase of cyclin D1 expression with progression from dysplasia to invasive cancers, which is agreement with our result. This slight increase of cyclin D1 in cervical cancer may be associated with the involvement of cyclin D1 in the acquisition of invasive potential [38]. Recent studies have revealed the specific function of cyclin D1 in cell migration [3940]. Cyclin D1 may promote cancer cell migration by inhibiting the Rho/Rho-associated protein kinase signaling and matrix deposition of TSP–1, an extracellular matrix protein [40]. Taken together, cyclin D1 appears to be differently functioning in precancerous and cancerous lesions of the cervix.
Recent studies have shown GSK3β as a promising therapeutic target in oral, renal, bladder, pancreatic, and colon cancers and leukemia [151719202223]. Our data also identified increased expression of GSK3β with severity of the disease. But considering its functional complexity and diversity, particular cautions are needed when adopting inhibitor of GSK3β for clinical trials in cancer patients. This pluripotent kinase indeed causes degradation of cyclin D1 thereby suppressing signals that promote cancer but may also simultaneously block cell cycle inhibitors like p27 and p21, which play a key role in disrupting cell cycle [11]. Therefore, blocking these factors may cause opposite effect leading to unwanted proliferation.
In conclusion, our study showed the expression of GSK3β increased with disease progress from CIN to cancer and decreased expression of cyclin D1 in CIN3 and cancer compared to CIN1. Also GSK3β expression was significantly associated with the histologic type of cervical cancer, especially SCC. These results suggest a possible involvement of GSK3β and cyclin D1 in cervical carcinogenesis and would provide useful information when considering GSK3β inhibitor for clinical trials in cervical cancer.
Figures and Tables
Fig. 1
Representative immunohistochemical staining (×400) of glycogen synthase kinase 3β in cervical intraepithelial neoplasia (CIN) 1 (A), CIN3 (C), and squamous cell carcinoma (E), and of cyclin D1 in CIN1 (B), CIN3 (D), and squamous cell carcinoma (F).
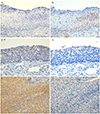
Fig. 2
Squamous cell carcinoma showed moderate to strong immunoreactivity (×400) to glycogen synthase kinase 3β (A), while adenocarcinoma showed weak or negative immunoreactivity (×400) (B).

References
1. HPV Information Centre. Human papillomavirus and related diseases report [Internet]. Barcelona: ICO Information Centre on HPV and Cancer;2016. 2016 Jan 28. Available from: http://www.hpvcentre.net/statistics/reports/XWX.pdf.
2. zur Hausen H. Papillomaviruses causing cancer: evasion from host-cell control in early events in carcinogenesis. J Natl Cancer Inst. 2000; 92:690–698.
3. Bahnassy AA, Zekri AR, Saleh M, Lotayef M, Moneir M, Shawki O. The possible role of cell cycle regulators in multistep process of HPV-associated cervical carcinoma. BMC Clin Pathol. 2007; 7:4.
4. Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci (Lond). 2006; 110:525–541.
5. Howley PM, Scheffner M, Huibregtse J, Munger K. Oncoproteins encoded by the cancer-associated human papillomaviruses target the products of the retinoblastoma and p53 tumor suppressor genes. Cold Spring Harb Symp Quant Biol. 1991; 56:149–155.
6. Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer. 2010; 10:550–560.
7. Perez-Plasencia C, Vazquez-Ortiz G, Lopez-Romero R, Pina-Sanchez P, Moreno J, Salcedo M. Genome wide expression analysis in HPV16 cervical cancer: identification of altered metabolic pathways. Infect Agent Cancer. 2007; 2:16.
8. Vandermark ER, Deluca KA, Gardner CR, Marker DF, Schreiner CN, Strickland DA, et al. Human papillomavirus type 16 E6 and E 7 proteins alter NF-kB in cultured cervical epithelial cells and inhibition of NF-kB promotes cell growth and immortalization. Virology. 2012; 425:53–60.
9. Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003; 116(Pt 7):1175–1186.
10. Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004; 29:95–102.
11. McCubrey JA, Steelman LS, Bertrand FE, Davis NM, Sokolosky M, Abrams SL, et al. GSK-3 as potential target for therapeutic intervention in cancer. Oncotarget. 2014; 5:2881–2911.
12. Sears R, Nuckolls F, Haura E, Taya Y, Tamai K, Nevins JR. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev. 2000; 14:2501–2514.
13. Tullai JW, Chen J, Schaffer ME, Kamenetsky E, Kasif S, Cooper GM. Glycogen synthase kinase-3 represses cyclic AMP response element-binding protein (CREB)-targeted immediate early genes in quiescent cells. J Biol Chem. 2007; 282:9482–9491.
14. Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998; 12:3499–3511.
15. Ougolkov AV, Fernandez-Zapico ME, Bilim VN, Smyrk TC, Chari ST, Billadeau DD. Aberrant nuclear accumulation of glycogen synthase kinase-3beta in human pancreatic cancer: association with kinase activity and tumor dedifferentiation. Clin Cancer Res. 2006; 12:5074–5081.
16. Karrasch T, Spaeth T, Allard B, Jobin C. PI3K-dependent GSK3ß(Ser9)-phosphorylation is implicated in the intestinal epithelial cell wound-healing response. PLoS One. 2011; 6:e26340.
17. Mishra R. Glycogen synthase kinase 3 beta: can it be a target for oral cancer. Mol Cancer. 2010; 9:144.
18. Mishra R, Nagini S, Rana A. Expression and inactivation of glycogen synthase kinase 3 alpha/beta and their association with the expression of cyclin D1 and p53 in oral squamous cell carcinoma progression. Mol Cancer. 2015; 14:20.
19. Shakoori A, Mai W, Miyashita K, Yasumoto K, Takahashi Y, Ooi A, et al. Inhibition of GSK-3 beta activity attenuates proliferation of human colon cancer cells in rodents. Cancer Sci. 2007; 98:1388–1393.
20. Naito S, Bilim V, Yuuki K, Ugolkov A, Motoyama T, Nagaoka A, et al. Glycogen synthase kinase-3beta: a prognostic marker and a potential therapeutic target in human bladder cancer. Clin Cancer Res. 2010; 16:5124–5132.
21. Cao Q, Lu X, Feng YJ. Glycogen synthase kinase-3beta positively regulates the proliferation of human ovarian cancer cells. Cell Res. 2006; 16:671–677.
22. Bilim V, Ougolkov A, Yuuki K, Naito S, Kawazoe H, Muto A, et al. Glycogen synthase kinase-3: a new therapeutic target in renal cell carcinoma. Br J Cancer. 2009; 101:2005–2014.
23. Miyashita K, Kawakami K, Nakada M, Mai W, Shakoori A, Fujisawa H, et al. Potential therapeutic effect of glycogen synthase kinase 3beta inhibition against human glioblastoma. Clin Cancer Res. 2009; 15:887–897.
24. Kato J, Matsushime H, Hiebert SW, Ewen ME, Sherr CJ. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993; 7:331–342.
25. Leone G, DeGregori J, Jakoi L, Cook JG, Nevins JR. Collaborative role of E2F transcriptional activity and G1 cyclindependent kinase activity in the induction of S phase. Proc Natl Acad Sci U S A. 1999; 96:6626–6631.
26. Bartkova J, Lukas J, Muller H, Strauss M, Gusterson B, Bartek J. Abnormal patterns of D-type cyclin expression and G1 regulation in human head and neck cancer. Cancer Res. 1995; 55:949–956.
27. Cheung TH, Yu MM, Lo KW, Yim SF, Chung TK, Wong YF. Alteration of cyclin D1 and CDK4 gene in carcinoma of uterine cervix. Cancer Lett. 2001; 166:199–206.
28. Ma C, Wang J, Gao Y, Gao TW, Chen G, Bower KA, et al. The role of glycogen synthase kinase 3beta in the transformation of epidermal cells. Cancer Res. 2007; 67:7756–7764.
29. Skomedal H, Kristensen GB, Lie AK, Holm R. Aberrant expression of the cell cycle associated proteins TP53, MDM2, p21, p27, cdk4, cyclin D1, RB, and EGFR in cervical carcinomas. Gynecol Oncol. 1999; 73:223–228.
30. Cho NH, Kim YT, Kim JW. Correlation between G1 cyclins and HPV in the uterine cervix. Int J Gynecol Pathol. 1997; 16:339–347.
31. Bae DS, Cho SB, Kim YJ, Whang JD, Song SY, Park CS, et al. Aberrant expression of cyclin D1 is associated with poor prognosis in early stage cervical cancer of the uterus. Gynecol Oncol. 2001; 81:341–347.
32. Rath G, Jawanjal P, Salhan S, Nalliah M, Dhawan I. Clinical significance of inactivated glycogen synthase kinase 3β in HPV-associated cervical cancer: Relationship with Wnt/β-catenin pathway activation. Am J Reprod Immunol. 2015; 73:460–478.
33. Choi SK, Hong YO, Lee WM, Kim EK, Joo JE, Kim DW, et al. Overexpression of PI3K-p110α in the progression of uterine cervical neoplasia and its correlation with pAkt and DJ-1. Eur J Gynaecol Oncol. 2015; 36:389–393.
34. de Groot RP, Auwerx J, Bourouis M, Sassone-Corsi P. Negative regulation of Jun/AP-1: conserved function of glycogen synthase kinase 3 and the Drosophila kinase shaggy. Oncogene. 1993; 8:841–847.
35. Manoukian AS, Woodgett JR. Role of glycogen synthase kinase-3 in cancer: regulation by Wnts and other signaling pathways. Adv Cancer Res. 2002; 84:203–229.
36. Nichols GE, Williams ME, Gaffey MJ, Stoler MH. Cyclin D1 gene expression in human cervical neoplasia. Mod Pathol. 1996; 9:418–425.
37. Lukas J, Muller H, Bartkova J, Spitkovsky D, Kjerulff AA, Jansen-Durr P, et al. DNA tumor virus oncoproteins and retinoblastoma gene mutations share the ability to relieve the cell’s requirement for cyclin D1 function in G1. J Cell Biol. 1994; 125:625–638.
38. Koay MH, Crook M, Stewart CJ. Cyclin D1, E-cadherin and beta-catenin expression in FIGO Stage IA cervical squamous carcinoma: diagnostic value and evidence for epithelial-mesenchymal transition. Histopathology. 2012; 61:1125–1133.
39. Casimiro MC, Crosariol M, Loro E, Li Z, Pestell RG. Cyclins and cell cycle control in cancer and disease. Genes Cancer. 2012; 3:649–657.
40. Li Z, Wang C, Prendergast GC, Pestell RG. Cyclin D1 functions in cell migration. Cell Cycle. 2006; 5:2440–2442.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download