1. Hill JM, Loeb E, MacLellan A, Hill NO, Khan A, King JJ. Clinical studies of platinum coordination compounds in the treatment of various malignant diseases. Cancer Chemother Rep. 1975; 59:647–659.
2. Hill JM, Speer RJ. Organo-platinum complexes as antitumor agents (review). Anticancer Res. 1982; 2:173–186.
3. Matsuyama R, Reddy S, Smith TJ. Why do patients choose chemotherapy near the end of life? A review of the perspective of those facing death from cancer. J Clin Oncol. 2006; 24:3490–3496.
4. Bartelink H, Schellens JH, Verheij M. The combined use of radiotherapy and chemotherapy in the treatment of solid tumours. Eur J Cancer. 2002; 38:216–222.
5. Arany I, Safirstein RL. Cisplatin nephrotoxicity. Semin Nephrol. 2003; 23:460–464.
6. Dos Santos NA, Carvalho Rodrigues MA, Martins NM, dos Santos AC. Cisplatin-induced nephrotoxicity and targets of nephroprotection: an update. Arch Toxicol. 2012; 86:1233–1250.
7. Sadzuka Y, Shoji T, Takino Y. Effect of cisplatin on the activities of enzymes which protect against lipid peroxidation. Biochem Pharmacol. 1992; 43:1872–1875.
8. Appenroth D, Frob S, Kersten L, Splinter FK, Winnefeld K. Protective effects of vitamin E and C on cisplatin nephrotoxicity in developing rats. Arch Toxicol. 1997; 71:677–683.
9. Naziroglu M, Karaoglu A, Aksoy AO. Selenium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal, liver and lens tissues in rats. Toxicology. 2004; 195:221–230.
10. Atessahin A, Ceribasi AO, Yuce A, Bulmus O, Cikim G. Role of ellagic acid against cisplatin-induced nephrotoxicity and oxidative stress in rats. Basic Clin Pharmacol Toxicol. 2007; 100:121–126.
11. Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010; 2:1231–1246.
12. Lambert JD, Elias RJ. The antioxidant and pro-oxidant activities of green tea polyphenols: a role in cancer prevention. Arch Biochem Biophys. 2010; 501:65–72.
13. Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003; 43:89–143.
14. Yang CS, Wang X. Green tea and cancer prevention. Nutr Cancer. 2010; 62:931–937.
15. El-Mowafy AM, Salem HA, Al-Gayyar MM, El-Mesery ME, El-Azab MF. Evaluation of renal protective effects of the green-tea (EGCG) and red grape resveratrol: role of oxidative stress and inflammatory cytokines. Nat Prod Res. 2011; 25:850–856.
16. Sahin K, Tuzcu M, Gencoglu H, Dogukan A, Timurkan M, Sahin N, et al. Epigallocatechin-3-gallate activates Nrf2/HO-1 signaling pathway in cisplatin-induced nephrotoxicity in rats. Life Sci. 2010; 87:240–245.
17. Khan SA, Priyamvada S, Khan W, Khan S, Farooq N, Yusufi AN. Studies on the protective effect of green tea against cisplatin induced nephrotoxicity. Pharmacol Res. 2009; 60:382–391.
18. Tate SS, Meister A. Gamma-glutamyl transpeptidase from kidney. Methods Enzymol. 1985; 113:400–419.
19. Tenenhouse HS, Scriver CR, Vizel EJ. Alkaline phosphatase activity does not mediate phosphate transport in the renal-cortical brush-border membrane. Biochem J. 1980; 190:473–476.
20. Fatima S, Arivarasu NA, Mahmood R. Vitamin C attenuates cisplatin-induced alterations in renal brush border membrane enzymes and phosphate transport. Hum Exp Toxicol. 2007; 26:419–426.

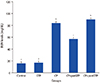
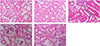
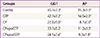
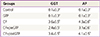




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download