Pulmonary hypertension is a rare and potentially life-threatening complication of SLE, and 5 cases has been previously documented in pregnancy.1-3 Only one case was alive after delivery,1 other 4 cases died after delivery.1-3 Pulmonary hypertension is reported in 5% to 14% of patients with SLE. Overall mortality is 25~50% two years after diagnosis of pulmonary hypertension.4 The most common symptoms of pulmonary hypertension in patients with SLE are fatigue, dyspnea and syncope. Raynaud's phenomena is present in 75% of patients with pulmonary hypertension related to SLE, compared with 25% in patients with SLE without pulmonary hypertension.5 Echocardiography reveals right ventricular hypertrophy and dilatation. Pulmonary hypertension in patients with SLE is associated with intimal hyperplasia, smooth muscle hypertrophy and medial thickening, similar to the changes seen in primary pulmonary hypertension.4 The development of pulmonary hypertension is not necessarily consistent with the stage of the SLE and severity of the lupus activity. The diagnosis is frequently delayed because the symptoms tend to occur late by which time the pulmonary artery pressure may be severely elevated and cardiac output reduced.1
The case presented here is that of a pregnant woman with SLE and pulmonary hypertension which has not been hitherto documented in Korea.
Case Report
A 25-year-old multiparous woman, was referred to us at 22 weeks of gestation. The chief complaints were dyspnea, fatigue, anorexia, fevers and chills, and pain sensation of both legs for 2 weeks. A cyanotic face and clubbed fingers were noted at admission.
The patient's history revealed no previous disease diagnosis including immunologic diseases. Two years previously, the patient had uneventfully given birth a 2.4 kg baby at term.
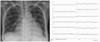
Chest X-ray revealed cardiomegaly (cardiothoracic ratio >0.66) and mild pulmonary congestion (Fig. 1A). Electrocardiogram showed low voltage with normal sinus rhythm (Fig. 1B). An echocardiogram showed an elevated right ventricular systolic pressure 87.4 mmHg (normal 20~25 mmHg) with mild tricuspid regurgitation, an elevated right atrial pressure of 15 mmHg (normal 0~8 mmHg), right ventricular and atrial dilatation, and a small amount of pericardial effusion. Left-sided cardiac function was normal, and intracardiac shunt was not seen. Computed tomography (CT) of the lung was performed to exclude pulmonary thrombosis or embolus as a cause of pulmonary hypertension. CT did not reveal occlusion or thrombus of pulmonary arteries, and showed bilateral pleural effusion and pericardial effusion. Complete blood count showed leucopenia and anemia (white blood cell count (WBC); 1,250/dL (66.3% neutrophil, 17.2% lymphocyte), hemoglobin (Hb); 7.9 g/dL, hematocrit (Hct); 24.4, platelet count 138,000/µL). Urine analysis showed 2+ urine protein. The liver enzyme aspartate aminotransferase/alanine trasnsaminase (AST/ALT) were slightly increased (114/43 U/L) and blood urea nitrogen and creatinine (BUN/Cr) was normal range (5.0/7.0 mg/day) (Table 1). Immunologic laboratory results were FANA (+) (1:1600, homogenous type), Anti-ds DNA (+) (25.37), Lupus anticoagulant (+), Anti-RNP (3+), coombs direct test (+), hypocompletenemia (C3/C4: 39/5) (Table 2).
On fetal sonogram, fetal estimated weight was about the 3rd percentile size (0.42 kg) and early diastolic notch sign of bilateral uterine arteries was noted (Fig. 2A, B). Resistant index (RI) of the umbilical arteries was 0.69 (Fig. 2C) and RI of middle cerebralartery (MCA) was 0.82 (Fig. 2D), which were both showed within normal range. No anomalies were found on the fetal echocardiogram. Based on these findings, the patient was diagnosed with severe pulmonary hypertension secondary to SLE in pregnancy, with intrauterine growth restriction (IUGR)
Oral prednisolone (15 mg/day) and hydroxychloroquine (400 mg/day) was immediately commenced.
An echocardiogram obtained 1-week later showed reduced right ventricular systolic pressure (59 mmHg). The patient was discharged on day 27 on medication of prednisolone and hydroxychloroquine. She visited every week at department of obstetrics and rheumatology.
During the remainder of pregnancy, other than mild dyspnea, the patient's condition and course were unremarkable. A follow-up sonography revealed an estimated fetal weights of 1.16 kg at 29 weeks of gestation (10th percentile), 1.39 kg at 31 weeks of gestation (10th percentile) and 1.63 kg at 33 weeks of gestation (3rd percentile). No evidence of fetal compromise was evident. RI of umbilical artery at 33 weeks of gestation (0.54) was within the normal range compatible with gestation (Fig. 2E), which shows an increase in diastolic velocities and a decline in RI (=0.69) compared as that at 24 weeks of gestation. However, early diastolic notch of uterine artery persisted and the diastolic velocity of MCA increased (RI=0.66) (Fig. 2F).
The initial plan was to allow the pregnancy to proceed to at most 34 weeks and then induce labor for vaginal delivery. However, upon the insistence of the patient and her husband, the pregnancy was extended to 38 weeks while ensuring that the health of both patient and fetus was maintained. At the time of admission for labor induction, patient's blood pressure was 120/80 mmHg and some mild dyspnea was noted. The dilatation of cervix was about 2 cm. The degree of facial cyanosis and finger clubbing had not changed. Chest X-ray did not demonstrate cardiomegaly or other abnormalities. Laboratory results were improved from the initial laboratory data. Hb 10.0 g/dL, WBC 5,500/dL, platelet count 352,000/µL, AST/ALT 27/24 IU/L, C3 119 mg/dL, anti-ds-DNA antibody 17.89 (Table 2).
Low dose oxytocin infusion was used for augmentation of labor and epidural blockade was done for analgesia. Labor was uneventful and a healthy female neonate weighing 2.40 kg was delivered vaginally. Apgar scores at 1 and 5 min were 7 and 5, respectively, and the pH of umbilical artery and vein was 7.290.
The patient was managed on intensive care unit and after 72 hours she was transferred back to the maternity unit. The patient's vital signs were normal and there was not profuse vaginal bleeding. The patient made an uneventful postpartum recovery and was discharged 6-days after delivery on an oral regimen of prednisolone and hydroxychloroquine. The pathology of placenta showed acute chorioamnionitis with microcalcification. Echocardiographic studies revealed dilatation of the right ventricle regurgitation of mild tricuspid was noted. PA systolic pressure of Right ventricle decreased from 87.5 mmHg in pregnancy to 50 mmHg at postpartum. Follow-up examination over the next 3-months conducted at rheumatology-cardiologic clinic and the obstetric clinic revealed no obvious deterioration.
Discussion
Respiratory involvement in SLE is not as well known as the cutaneous, rheumatological and renal manifestations. It can be classified in 5 groups based on the anatomy: pleural involvement, infiltrating pneumonia, airways involvement, vascular involvement, muscular and diaphragmatic involvement. Pulmonary hypertension represents vascular involvement.6 Pulmonary hypertension is present when systolic pulmonary arterial pressure exceeds 30 mmHg or the mean pulmonary artery pressure exceed 20 mmHg.7 The initial systolic pulmonary arterial pressure of this case's patient was 87.4 mmHg. The causes of secondary pulmonary hypertension in pregnancy were Takayasu's arteritis, pulmonary vasculitis of connective tissue such as SLE or scleroderma, sickle cell disease, chronic pulmonary thromboembolism, hepatitis, dwarfism with congenital hypothyroidism and peripheral pulmonary stenoses.8 We performed CT of the lung to exclude pulmonary thrombosis or embolus as a cause of pulmonary hypertension.
The pathogenesis of pulmonary hypertension in SLE has not elucidated clearly yet. However, the frequently observed association with Raynaud's phenomenon is consistent with a chronic and diffuse vasospastic condition which leads to muscular necrosis and secondary inflammation.9 The mortality of pulmonary hypertension in SLE was exceeds 50%, and often involves sudden death. Five cases of pulmonary hypertension associated with SLE have been previously documented in pregnancy.1-3 All but one delivery ended in death within 96 h postpartum.1 The time of highest risk for death from pulmonary hypertension is immediately after delivery or within the first 72 h postpartum. The reason of highest risk for maternal death in the first 48~72 hours after delivery may be related to the rise in the cardiac output and total blood volume during the pregnancy and the immediate postpartum period. The heart has the added load of an increased cardiac output with a fixed vascular resistance in the first 48~72 hours after delivery. In a patient with pulmonary hypertension, the increasing cardiac output and the already increased pulmonary vascular resistance above normal may result in reduced left ventricular filling, hypoxic state, and finally acute right heart failure as the cause of sudden maternal death.1,3
SLE and/or pulmonary hypertension are frequently associated with IUGR. IUGR has been reported in up to 33% of cases of pulmonary hypertension10 and 40% of cases of SLE.11 All five neonates, who previously documented in the cases of pulmonary hypertension associated with SLE in pregnancy, were IUGR.1-3 Antiphospholipid antibodies, hypocomplementemia correlates with IUGR in SLE.1,12 Bilateral uterine artery notches at 22~24 weeks gestation in women with positive lupus anticoagulant and/or antiphospholipid antibody is a diagnostically accurate indicator of adverse pregnancy outcomes that including IUGR, preeclampsia, placental abruption and intrauterine fetal death.13,14 Thrombogenic action of lupus anticoagulant and/or antiphospholipid antibody leads to decreased placental perfusion and subsequent infarction. The antiphospholipid antibody-mediated inhibition of trophoblastic invasion and antiphospholipid antibody-mediated vasculopathy in placental bed arteries result in abnormal uterine artery waveforms.14 In the present case, bilateral uterine artery notches were observed at gestation week 22 and were maintained until term. And hypocomplementenia and positive lupus anticoagulant antibody were shown in the immunologic laboratory data. At term, MCA Doppler velocities showed increased pattern that means brain sparing effect in IUGR.15 The neonate's birth weight corresponded to about the 3rd percentile,16 fortunately other adverse pregnancy outcome such as preeclampsia or intrauterine death did not occur.
Vaginal delivery may be appropriate if adequate pain control is provided and fetal distress does not develop.1 Oxytocin should be used with care because of its systemic vasodilatation. However, low dose oxytocin infusion used for augmentation of labor dose not seem to be have detrimental effects.17 Adequate analgesia is necessary for preventing from maternal hypoxia and acidosis developed due to labor pain. The maternal intensive care should be necessary for 72 h after birth because this period is critical time for maternal death. Pulmonary vasodilators such as nitric oxide and prostaglandin have some evidence that treatment with those medication improve symptoms and exercise capacity in the non-pregnant patient.18,19 However, there are a few cases in the use of prostaglandin or nitric oxide in pregnancy.20,21
In summary, very few reports have been published on lupus-related pulmonary hypertension in pregnancy. Most of the reported cases ended in the early postpartum death. Fortunately, in the present case, delivery was uneventful and both the patient and the infant continue to thrive. It is hoped that early diagnosis and admission and individualized care will lead more often to successful outcome.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download