Abstract
Clear cell adenocarcinoma (CCAC) of uterine cervix is very rare tumor and only 4%-9% of entire adenocarcinoma appears to be diagnosed as clear cell type. Risk factors and pathogenesis of this disease are not exactly revealed. The intrauterine exposure to diethylstilbestrol (DES) and associated non-steroidal estrogen during pregnancy before 18 weeks is the only known risk factor, and also hormonal changes or genetic causes are suggested as the risk factors. We report a case of CCAC in the uterine cervix of 52-year-old virgin who had never been exposed to DES, with a brief review of related literature.
Clear cell adenocarcinoma (CCAC) of the uterine cervix is a rare disease accounting for only 4% of all adenocarcinomas of the uterine cervix. Though the etiology and pathogenesis are not clear, several studies have linked the occurrence of vaginal CCAC in young women with intrauterine exposure to the synthetic nonsteroidal estrogenic hormone, diethylstilbestrol (DES). Other factors reported to be associated with the development of CCAC are microsatellite instability, human papillomavirus infection, bcl-2 protein overexpression, and p53 gene mutation. CCAC is refractory to chemotherapy or radiation therapy, and its prognosis is poorer than that of squamous cell carcinoma of the same organ. We experienced a case of CCAC in the uterine cervix of 52-year-old virgin who had never been exposed to DES and report this case with a brief review of related literature.
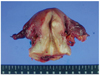
A 52-year-old woman had suffered vaginal spotting for about 3 months. She visited a local gynecologist and liquid based cytology (Papanicolaou smear) was done at uterine cervix by the gynecologist. The cytology showed atypical endocervical cells that favored neoplastic changes, which then she was referred to our outpatient department for further evaluation and management. She had no history of coitus and had never been exposed to in utero exogenous steroid hormone. The vaginal examination and inspection showed cauliflower-like mass of the cervix (Fig. 1). On rectal examination, the left parametrium was free and right parametrium was moderately thickened. High-risk human papillomavirus (hrHPV) test at uterine cervix done by hybrid capture II was negative. Histological diagnosis by punch biopsy was CCAC of the uterine cervix. Magnetic resonance imaging showed a solid mass, 1.6×2.9×2.5 cm in diameter (Fig. 2). This solid mass was protruding from the posterior lip of the cervix and extending to the upper vagina. Positron emission tomography-computed tomography was also performed, and there was evidence of tumor expansion neither to parametrium nor to endometrium. No involvement of pelvic and para-aortic lymph nodes was found. Parameters from blood chemistry studies and the levels of tumor markers were all within normal limits (carcinoembryonic antigen, 0.8 ng/mL; CA-125, 15.85 U/mL; CA 19-9, 10.7 U/mL; squamous-cell carcinoma, 0.6 ng/mL; alpha-fetoprotein, 7.9 ng/mL; beta-human chorionic gonadotropin, <1.2 mIU/mlL). There were no abnormal findings in intravenous pyelography, cystoscopy, and colonoscopy except for hyperplastic polyp in colon. Under the diagnosis of stage IIA cervical cancer, a radical abdominal hysterectomy with bilateral salpingo-oophorectomy and systemic pelvic lymphadenectomy was performed because the patient had no history of coitus and the vagina was too narrow. Macroscopic examination of the specimen revealed the irregular and hemorrhagic mass, 2.6 cm in diameter, infiltrating to the cervical wall (Fig. 3). Microscopically, the cancer cells had invaded the cervical wall to a depth of 6.0mm, with clear vaginal resection margin (Fig. 4) and there was no invasion of the endometrium or parametrium, also, no evidence of metastasis in 36 lymph nodes. The results of immunohistochemical stains are carcinoembryonic antigen (-), cytokeratin (+), vimentin (-), estrogen receptor (-) and progesterone receptor (-). After the surgery, she was doing well and was discharged 12 days after operation with no major complications. She did not receive any chemotherapy or radiation therapy and has visited our outpatient department twice a month for first 2 months. Then, she was planned to visit our clinic after 3 months.
Most cancers of the uterine cervix are squamous cell carcinomas, but the relative and absolute incidence of adenocarcinoma is rising in recent years and adenocarcinoma now accounts for about 20% of incidental invasive cervical cancers in screened populations worldwide [1]. CCAC of the cervix is an uncommon tumor, accounting for about 4% of cervical adenocarcinoma [2]. It is most widely associated with DES use in the 1970s but with the cessation of DES use, the incidence has fallen, and there only have been a few reports of small series in recent years.
CCAC has a bimodal age distribution. The first peak occurs in women who are 17 to 37 years of age (mean age 26 years), and the second peak occurs in women who are 44 to 88 years of age (mean age 71 years). The former group includes most of DES-exposed women, and the latter is constituted mostly by those without a history of DES exposure [3].
DES has been considered as a teratogen which crosses placental barrier [4]. During 4th and 18th weeks of normal development, the Müllerian ducts of the embryo extend caudally and fuse to form a solid structure, the uterovaginal primordium. Squamous cells arising in the epithelium of the urogenital sinus invade the primordium from below and completely replace the Müllerian mucosa up to the level of the external cervical os. DES administration before 18th weeks of pregnancy stimulates the persistence of the Müllerian epithelium or inhibits its replacement by squamous epithelium in the vagina [5].
DES-exposed women have a 40-fold increased risk of developing CCAC, resulting in a cumulative indence of 0.1%-0.2%. As this tumor is still very rare in DES-exposed women, DES is suggested to be an incomplete carcinogen. Although 60% of CCAC are detected in DES-exposed women, 40% develop in unexposed women, indicating the involvement of alternative etiologic factors. A factor of interest might be a transforming infection with a hrHPV, the major causative factor in almost all cervical squamous cell carcinoma and adenocarcinoma [6]. Only few studies have explored the association between hrHPV and CCAC. In this small studies hrHPV positivity varied between 0% and 100%, thereby hampering any conclusion to be made about its potential causal role [7,8]. In our case, hrHPV test done by hybrid capture II was negative.
Waggoner et al investigated p53 protein over-expression and also performed mutational analysis of the p53 gene using polymerase chain reaction (PCR) in clear cell carcinoma of the vagina and cervix. Over expression of the p53 protein was noted in 4 of 21 cases on immunohistochemical staining. The p53 gene analysis showed no mutation. However, 87% of cases showed over-expression of bcl-2 protein, and those tumors had lower apoptotic indices. So, they concluded that abnormalities of the replication of damaged DNA, involved in the mechanism of apoptosis are a cause of clear cell carcinoma of the vagina and cervix. They also hypothesized that bcl-2 over-expression could inhibit p53-mediated apoptosis and by the means which CCAC could arise in the absence of a p53 gene mutation [9]. Boyd et al. [10] described the genetic characteristics of 24 cases of clear cell carcinoma of the vagina and cervix. No mutations were found in K-ras, H-ras, WT1, or the estrogen receptor gene. Although some cases of DES-associated clear cell carcinoma demonstrated over-expression of p53 protein on immunohistochemical staining, these cases did not exhibit a p53 mutation in exons 4 to 9 using single strand conformation polymorphism analysis by PCR amplification of genomic DNA. They also found widespread microsatellite instability in all DES-exposed tumors and in 50% of tumors that were not associated with DES exposure. This led them to conclude that the induction of genomic instability may be an important mechanism of DES-induced carcinogenesis [10].
CCAC typically causes prolonged vaginal bleeding that, when occurring in a child or young woman, is often misunderstood as precocious puberty or anovulatory bleeding. Routine vaginal cytology is often negative, and the tumors frequently cannot be palpated on examination, making diagnosis challenging. The possibility of a vaginal tumor must be considered in the differential diagnosis of prolonged vaginal bleeding, especially if the bleeding does not respond to hormone therapy in cases of adult women [11]. Most CCACs are of Mullerian origin. The tumors may involve any portion of the vagina, uterine cervix, or both. Approximately 60% of lesions are confined to the vagina, particularly those involving the anterior wall of the upper third, which is most frequent site of vaginal adenosis [12]. These areas contain mucinous columnar cell metaplasia and columnar cells of the tubo-endometrial type, with or without cilia, resembling the lining cells of the normal endometrium or fallopian tube. The finding of these elements in our patient supports the preneoplastic nature of vaginal adenosis in the tumorigenic pathway of CCAC. Most cervical tumors are superficial and invade only a few millimeters into the vaginal or cervical wall. These lesions often occur in older women in whom there are also areas of endometriosis [13].
Most women had early stage (stage I-II) disease and our patient was stage IIA. In a Dutch series of 88 women, 76 (88.5%) had stage I-II disease [3]. The primary treatment of early disease usually involved radical surgery with pelvic±para-aortic lymphadenectomy, with outcomes comparable to other types of cervical adenocarcinoma [14,15].
Important parameters for the determination of prognosis of CCAC patients are stage, tumor size, growth pattern, nuclear atypia, and mitotic activity [1,4,5]. In the current study, an unfavorable prognosis corresponded with high stage, large tumor size, high grade of nuclear atypia, and high mitotic activity. The tubulo-cystic growth pattern corresponded with a better prognosis than the solid or mixed growth pattern.
Metastases can occur even when the tumor is very small. All local recurrences occur within 3 years of initial diagnosis. Recently, a familial chromosome translocation has been described involving chromosome 3 and 6 [46, XX, t(3:6)(q29;q23)] in mother and her DES-exposed daughter [3]. Human papillomavirus (HPV) type 31 DNA also has been identified in CCAC, but the oncogenic potential of this HPV subtype is low and, hence, it is thought unlikely to be associated with the development of CCAC [10].
Though there have been many things to be proved about CCAC, we need more cases presented to evaluate the natural history and to find out more effective treatment of this disease.
Figures and Tables
Fig. 1
Inspection by vaginal speculum showed cauliflower-like mass of cervix, filling up the entire vagina.
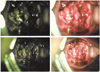
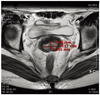
Fig. 2
Magnetic resonance imaging showed about 1.6×2.9 ×2.5 cm sized cervical mass protruding from posterior lip of cervix. There was no evidence of parametrial invasion, lymph node involvement or distant metastasis.
References
1. Herbst AL, Anderson D. Coppleson M, editor. Clear cell adenocarcinoma of cervix and vagina and DES-related abnormalities. Gynecologic oncology. 1992. 2nd ed. London: Churchill Livingstone;1–523.
2. Noller KL, Decker DG, Dockerty MB, Lanier AP, Smith RA, Symmonds RE. Mesonephric (clear cell) carcinoma of the vagina and cervix. A retrospective analysis. Obstet Gynecol. 1974. 43:640–644.
3. Hanselaar A, van Loosbroek M, Schuurbiers O, Helmerhorst T, Bulten J, Bernhelm J. Clear cell adenocarcinoma of the vagina and cervix. An update of the central Netherlands registry showing twin age incidence peaks. Cancer. 1997. 79:2229–2236.
4. Mittendorf R. Teratogen update: carcinogenesis and teratogenesis associated with exposure to diethylstilbestrol (DES) in utero. Teratology. 1995. 51:435–445.
5. Herbst AL, Robboy SJ, Scully RE, Poskanzer DC. Clear-cell adenocarcinoma of the vagina and cervix in girls: analysis of 170 registry cases. Am J Obstet Gynecol. 1974. 119:713–724.
6. Kocken M, Baalbergen A, Snijders PJ, Bulten J, Quint WG, Smedts F, et al. High-risk human papillomavirus seems not involved in DES-related and of limited importance in nonDES related clear-cell carcinoma of the cervix. Gynecol Oncol. 2011. 122:297–302.
7. Pirog EC, Kleter B, Olgac S, Bobkiewicz P, Lindeman J, Quint WG, et al. Prevalence of human papillomavirus DNA in different histological subtypes of cervical adenocarcinoma. Am J Pathol. 2000. 157:1055–1062.
8. Waggoner SE, Anderson SM, Van Eyck S, Fuller J, Luce MC, Herbst AL. Human papillomavirus detection and p53 expression in clear-cell adenocarcinoma of the vagina and cervix. Obstet Gynecol. 1994. 84:404–408.
9. Waggoner SE, Mittendorf R, Biney N, Anderson D, Herbst AL. Influence of in utero diethylstilbestrol exposure on the prognosis and biologic behavior of vaginal clear-cell adenocarcinoma. Gynecol Oncol. 1994. 55:238–244.
10. Boyd J, Takahashi H, Waggoner SE, Jones LA, Hajek RA, Wharton JT, et al. Molecular genetic analysis of clear cell adenocarcinomas of the vagina and cervix associated and unassociated with diethylstilbestrol exposure in utero. Cancer. 1996. 77:507–513.
11. Herbst AL, Scully RE. Adenocarcinoma of the vagina in adolescence. A report of 7 cases including 6 clear-cell carcinomas (so-called mesonephromas). Cancer. 1970. 25:745–757.
12. Matias-Guiu X, Lerma E, Prat J. Clear cell tumors of the female genital tract. Semin Diagn Pathol. 1997. 14:233–239.
13. Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med. 1971. 284:878–881.
14. Kaminski PF, Maier RC. Clear cell adenocarcinoma of the cervix unrelated to diethylstilbestrol exposure. Obstet Gynecol. 1983. 62:720–727.
15. Reich O, Tamussino K, Lahousen M, Pickel H, Haas J, Winter R. Clear cell carcinoma of the uterine cervix: pathology and prognosis in surgically treated stage IB-IIB disease in women not exposed in utero to diethylstilbestrol. Gynecol Oncol. 2000. 76:331–335.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download