Abstract
Background
Induced sputum analyses are widely utilized to evaluate airway inflammation in asthmatics. However, the values have not been examined in Korean adults.
Objective
The purpose of this study is to determine reference ranges for induced sputum eosinophils and their influencing factors in Korean adults.
Methods
A total of 208 healthy nonasthmatic adults were recruited. Sputum induction and processing followed the international standard protocols.
Results
Adequate sputum samples were successfully collected from 81 subjects (38.9%). The upper 90 percentile for sputum eosinophil was calculated as 3.5%. The median value of eosinophil count percentage was significantly higher in subjects with atopy than those without atopy (median, 1.6%; range, 0-11.0% vs. median, 0%; range 0-3.6%, p=0.030). However, no significant correlations were found with age, gender, body mass index, smoking status, blood eosinophil, or fractional exhaled nitric oxide levels.
Induced sputum analysis is one of representative tests to define airway inflammatory phenotype in asthma [1]. It has been standardized about a decade ago, and has been widely used in research and practice due to its reproducibility and less invasiveness [2, 3]. Induced sputum eosinophil is still one of the most useful biomarkers for asthma [1]; as it has clinical utility in phenotyping asthma or predicting the treatment response [1, 4, 5]. However, currently available reference levels (defined as more than 3% of eosinophil count in total cell count) have been derived from Western population studies [6, 7, 8, 9, 10], but not have been examined in Korean adults yet. Considering the potential heterogeneity in fractional exhaled nitric oxide (FeNO) levels among ethnic groups [11], induced sputum profiles also need to be examined specifically for Korean populations. In this study, we aimed to determine reference ranges for induced sputum eosinophil in healthy Korean adults.
Healthy adult volunteers were recruited by public posting from visitors to Seoul National University Hospital, Seoul, Korea. The recruitment was carried out from November 2010 to September 2011. Inclusion criteria were (1) age over 19-year-old, (2) no history of respiratory tract disease in previous 3 months, (3) no evidence of systemic infection in previous 3 months, and (4) absence of asthma related symptom and airway hyperresponsiveness (defined as methacholine provocative concentration causing 20% fall in forced expiratory volume during 1 second [PC20]≤16 mg/mL). Finally, 208 healthy volunteers were recruited, and all of them underwent induced sputum tests. All the participants were given written informed consents. The study protocol was approved by the Institutional Review Board of Seoul National University Hospital (IRB no. 1012-061-344).
First, we check baseline forced expiratory volume during 1 second (FEV1). They were pretreated with salbutamol 2 puff as a bronchodilator. Sputum was induced with hypertonic saline 4.5% via ultrasonic nebulizer, and was collected into a petri dish which was placed on the ice. FEV1 was checked every 5 minutes during 20 minutes. The sample was kept at 4℃, and sputum processing was performed within 4 hours of induction, with minor modifications of the European Respiratory Society standard protocol [12]. We removed saliva with the pipet and determined the volume of sputum. The equal volume of 0.01M dithioerythritol was added, and we gently mixed them. These were filtered with 100-µm cell strainer or mesh on 15-mL tube and centrifuged with 1,000 or 2,000 rpm for 10 minutes at 4℃. We aspirated supernatant 1 to 1.5 mL into a cryotube and freezed them at -20℃ or -80℃. The cell pellet was resuspended with 1-mL phosphate buffered saline (PBS). The volume of 10-µL suspension was added with 90-µL trypan blue. We determined cell counts and then dilute cell suspension with PBS to obtain 1×105 cells/mL. The cytospin was done at 500 rpm for 5 minutes at 4℃, and the Diff Quick stain was done. Finally we counted cells as %, except the epithelial cells.
The inhalant allergen skin prick test was performed to determine atopic status. Major inhalant allergens were evaluation, including Dermatophagoides pteronyssinus, Dermatophagoides farinae, cat, dog, tree mix, grass mix, ragweed, mugwort, cockroach, Alternaria spp., and Aspergillus fumigatus. Normal saline and histamine were used to negative and positive control. Atopy was defined if there are positive results (median wheal size ≥3 mm or larger than size of histamine, and median flare size ≥10 mm) to one or more allergen.
FeNO was measured by using NIOX MINO (Aerocrine, Solna, Sweden), according to the guidelines [13]. Briefly, they were asked to avoid medication or food intake which could interfere with the results. They were also instructed to avoid smoking or exercise within one hour before on the day of testing [14]. Then, they inhaled over 2-3 seconds to total lung capacity through NIOX filter, and exhaled as an upper airway pressure of 5-20 cm H2O. FeNO was measured during its plateau status at least for 3 seconds. As the lower detection limit was 5 parts per billion (ppb), the values <5 ppb were considered as 2.5 ppb. In this analysis, average levels of at least two acceptable measurements were utilized.
Statistical analyses were performed with SPSS ver. 10.0 (SPSS Inc., Chicago, IL, USA). As sputum inflammatory cell counts were not normally distributed, their distributions were described as median and ranges. The associations between cell counts and clinical factors were analyzed by the Mann-Whitney tests and the Spearman correlation tests. Results were considered significant when two-sided p values <0.05.
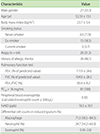
From 208 healthy volunteers who underwent induced sputum tests, 81 samples (38.9%) were eligible for analyses. The reasons for ineligibility were inadequate quality of sputum (n = 20), and failure to induce sputum (n = 107), presumably due to the lack of airway inflammation. Baseline characteristics of the study subjects were described in Table 1. Female were predominant (66.7%) and a mean age was 52.3 ± 13.1 years. Body mass index values were over 23.7 ± 3.4 kg/m2. Most of subjects (77.8%) were never-smoker and 20 subjects (31.3%) had atopy. There was no subject who had peripheral blood eosinophilia (≥500/µL). Most of subjects (72.8%) had low value of FeNO (<25 ppb) and the maximum value of FeNO was 49 ppb. Minimum value of sputum eosinophil was 0% and maximum value was 11%.
The distribution of induced sputum inflammatory cells in healthy subjects was described in Table 2. Macrophage and neutrophil were predominant cells in induced sputum. Macrophage had a median of 71.3% (range, 0-97.3%), and the upper 90 percentile of 89.2%. Neutrophil had a median of 24.7% (range, 0.7-100%), and the upper 90 percentile of 96.0%. Eosinophil had a median of 0% (range, 0-11%), and the upper 90 percentile of 3.5%. The distribution of induced sputum eosinophil was also presented in Fig. 1.
Associations of sputum eosinophil or neutrophil counts with demographic and clinical parameters were analyzed (Tables 3, 4). For eosinophils, subjects with atopy showed significantly higher counts than those without atopy. Distribution of induced sputum eosinophils (%) according to presence of atopy was presented in Fig. 2. However, no significant correlations were found with age, gender, smoking, body mass index, allergic rhinitis, peripheral blood eosinophil%, or FeNO levels. For neutrophils, no significant associations were found with any of these factors.
The present study reported, for the first time, the normal range of induced sputum eosinophils among Korean healthy adults. The range (3.5% as 90 percentile) was comparable to those from previous studies conducted in Western populations [6, 7].
Airway inflammation is an important pathogenesis underlying airway diseases such as asthma, chronic obstructive pulmonary disease, or chronic cough [15]. Therefore, to obtain airway sample is a mandatory step for both of research and clinical purposes. This is why, induced sputum test has gained a considerable popularity as a safe noninvasive alternative to bronchoscopy which could be at risk of exacerbations [1]. As, asthma is now considered as a heterogeneous entity with potentially various inflammatory phenotypes [16, 17], the necessity of the assessment is to continue.
In particular, sputum eosinophilia is a consistently important biomarker [18, 19]. Briefly, airway eosinophilia has been associated with airway obstruction, hyperresponsiveness, and corticosteroid response for asthma [20, 21, 22]. To determine this, the literature suggests the reference range as <2.4% [6, 7]. However, it should be noted that the reference values were obtained from relatively small numbers of studies conducted in Western populations [6, 7]. For FeNO analyses, the potential discrepancy between different ethnic or demographic groups have been suggested (frequently higher in Asians than Caucasians) [11, 14]. That was a motivation that we attempted to determine reference ranges in Korean adults in the present study.
We found that most of Korean healthy adults have sputum eosinophil <3.5%, which was slightly higher than previous reports [6, 7]. To our knowledge, no published literature directly compared different ethnic groups with large numbers of participants. We presume several possibilities for the discrepancy. First, the differences in clinical and demographic characteristics could have possibly influenced the results. Particularly, atopy could have been a significant factor, as also reported in the studies by Belda et al. [6]. Studies by Spanevello et al. [7], consisted of only nonatopics. The positive association between atopy and airway eosinophilia is a well-recognized finding [23], as both are Th2-mediated responses.
Our study population had older ages (mean, 52.3 years) and more females (66.7%) than these two reference studies (mean, 36 years and 38 years; and female, 55.2% and 47.9%, respectively) [6, 7]; however, age does not appear to correlate with sputum eosinophilia [6, 7]. Gender effects on sputum eosinophils remain elusive, but our lack of the difference might have been related to a low number of male participants; previous studies found females had 0.274% higher eosinophils than males [6].
Our high failure rates in sputum induction (61.1%) could also have been a bias factor. In the present study, 51.4% of participants could not expectorate sputum despite efforts. The rate of inadequate sample was 9.6%, which was comparable to total failure rates of 11.4-19% in previous studies [6, 7]. We processed the samples within 4 hours of induction, which was known to be acceptable [24]. We used 100-µm mesh for improving slide quality, which was larger than 40-µm nylon net filter in the international standard protocol [12]; however, the step of mesh filtering does not influence the sputum eosinophils [25].
Clinical utility of sputum neutrophilia and its consistency is still controversial, and was not fully discussed in this article. The distribution of sputum neutrophil% is wider than eosinophils, and more likely to have normal distributions [6, 7]. Airway neutrophilia has been associated with infection, irritant exposure, obesity, or sometimes with corticosteroid-resistant asthma [26] Among normal subjects, neutrophilia also appeared to be associated with older age [8].
The major limitation to our study is a sample size. We originally intended to analyze 150 or more normal subjects, but the success rate was lower than expected. Another limitation was the way of recruitment; participants were recruited from hospital visitors, which could not exclude the possibility of comorbidities other than asthma, respiratory diseases, or recent systemic infection. However, due to methodological considerations [24], sputum induction and processing should be performed with specialized instruments and very near to laboratories, making community-based sampling difficult.
In conclusions, the present study was the first attempt to determine the reference ranges of sputum eosinophils in Korean adults. Atopy was a clinical factor significantly associated with sputum eosinophilia.
Figures and Tables
Fig. 2
Distribution of induced sputum eosinophils of normal subjects according to presence of atopy (20 atopic subject and 43 nonatopic subjects).

Table 1
Baseline characteristics of healthy adults who successfully underwent induced sputum analyses (n = 81)
ACKNOWLEDGEMENTS
This study was supported by a grant of Korea Healthcare technology R&D projects, Ministry of Health & Welfare, Republic of Korea (A092076).
References
1. Davies AR, Hancox RJ. Induced sputum in asthma: diagnostic and therapeutic implications. Curr Opin Pulm Med. 2013; 19:60–65.
2. Pizzichini E, Pizzichini MM, Efthimiadis A, Evans S, Morris MM, Squillace D, Gleich GJ, Dolovich J, Hargreave FE. Indices of airway inflammation in induced sputum: reproducibility and validity of cell and fluid-phase measurements. Am J Respir Crit Care Med. 1996; 154(2 Pt 1):308–317.

3. Djukanović R, Sterk PJ, Fahy JV, Hargreave FE. Standardised methodology of sputum induction and processing. Eur Respir J Suppl. 2002; 37:1s–2s.
4. Brightling CE, McKenna S, Hargadon B, Birring S, Green R, Siva R, Berry M, Parker D, Monteiro W, Pavord ID, Bradding P. Sputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary disease. Thorax. 2005; 60:193–198.

5. Kitaguchi Y, Komatsu Y, Fujimoto K, Hanaoka M, Kubo K. Sputum eosinophilia can predict responsiveness to inhaled corticosteroid treatment in patients with overlap syndrome of COPD and asthma. Int J Chron Obstruct Pulmon Dis. 2012; 7:283–289.
6. Belda J, Leigh R, Parameswaran K, O'Byrne PM, Sears MR, Hargreave FE. Induced sputum cell counts in healthy adults. Am J Respir Crit Care Med. 2000; 161(2 Pt 1):475–478.

7. Spanevello A, Confalonieri M, Sulotto F, Romano F, Balzano G, Migliori GB, Bianchi A, Michetti G. Induced sputum cellularity. Reference values and distribution in normal volunteers. Am J Respir Crit Care Med. 2000; 162(3 Pt 1):1172–1174.
8. Thomas RA, Green RH, Brightling CE, Birring SS, Parker D, Wardlaw AJ, Pavord ID. The influence of age on induced sputum differential cell counts in normal subjects. Chest. 2004; 126:1811–1814.

9. Saha S, Brightling CE. Eosinophilic airway inflammation in COPD. Int J Chron Obstruct Pulmon Dis. 2006; 1:39–47.

10. Pavord ID, Brightling CE, Woltmann G, Wardlaw AJ. Non-eosinophilic corticosteroid unresponsive asthma. Lancet. 1999; 353:2213–2214.
11. Jo EJ, Song WJ, Kim TW, Park HW, Chang YS, Kim TB, Sim SH, Hur GY, Lee JH, Yoon HJ, Park HS, Cho NH, Moon HB, Min KU. Reference ranges and determinant factors for exhaled nitric oxide in a healthy Korean elderly population. Allergy Asthma Immunol Res. 2014; Forthcoming.

12. Efthimiadis A, Spanevello A, Hamid Q, Kelly MM, Linden M, Louis R, Pizzichini MM, Pizzichini E, Ronchi C, Van Overvel F, Djukanovic R. Methods of sputum processing for cell counts, immunocytochemistry and in situ hybridisation. Eur Respir J Suppl. 2002; 37:19s–23s.
13. American Thoracic Society. European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005; 171:912–930.
14. Song WJ, Kwon JW, Kim EJ, Lee SM, Kim SH, Lee SY, Kim SH, Park HW, Chang YS, Kim WK, Shim JY, Kim BJ, Kim HB, Song DJ, Jang GC, Jang AS, Park JW, Yoon HJ, Lee JS, Cho SH. Clinical application of exhaled nitric oxide measurements in a Korean population. Allergy Asthma Immunol Res. 2014; Forthcoming.

15. Ronchi MC, Piragino C, Rosi E, Amendola M, Duranti R, Scano G. Role of sputum differential cell count in detecting airway inflammation in patients with chronic bronchial asthma or COPD. Thorax. 1996; 51:1000–1004.

16. McGrath KW, Icitovic N, Boushey HA, Lazarus SC, Sutherland ER, Chinchilli VM, Fahy JV. Asthma Clinical Research Network of the National Heart, Lung, and Blood Institute. A large subgroup of mild-to-moderate asthma is persistently noneosinophilic. Am J Respir Crit Care Med. 2012; 185:612–619.

17. Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012; 18:716–725.

18. Brown HM. Treatment of chronic asthma with prednisolone; significance of eosinophils in the sputum. Lancet. 1958; 2:1245–1247.
19. Szefler SJ, Wenzel S, Brown R, Erzurum SC, Fahy JV, Hamilton RG, Hunt JF, Kita H, Liu AH, Panettieri RA Jr, Schleimer RP, Minnicozzi M. Asthma outcomes: biomarkers. J Allergy Clin Immunol. 2012; 129:3 Suppl. S9–S23.

20. Polosa R, Renaud L, Cacciola R, Prosperini G, Crimi N, Djukanovic R. Sputum eosinophilia is more closely associated with airway responsiveness to bradykinin than methacholine in asthma. Eur Respir J. 1998; 12:551–556.

21. Louis R, Lau LC, Bron AO, Roldaan AC, Radermecker M, Djukanovic R. The relationship between airways inflammation and asthma severity. Am J Respir Crit Care Med. 2000; 161:9–16.

22. Woodruff PG, Khashayar R, Lazarus SC, Janson S, Avila P, Boushey HA, Segal M, Fahy JV. Relationship between airway inflammation, hyperresponsiveness, and obstruction in asthma. J Allergy Clin Immunol. 2001; 108:753–758.

23. Busse WW, Sedgwick JB, Jarjour NN, Calhoun WJ. Eosinophils and basophils in allergic airway inflammation. J Allergy Clin Immunol. 1994; 94(6 Pt 2):1250–1254.

24. Efthimiadis A, Jayaram L, Weston S, Carruthers S, Hargreave FE. Induced sputum: time from expectoration to processing. Eur Respir J. 2002; 19:706–708.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download