1. Dominguez MS, Witherspoon DE, Gutmann JL, Opperman LA. Histological and scanning electron microscopy assessment of various vital pulp-therapy materials. J Endod. 2003; 29:324–333.

2. Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod. 1999; 25:197–205.

3. de Souza V, Holland R. Treatment of the inflamed dental pulp. Aust Dent J. 1974; 19:191–196.

4. Eskandarizadeh A, Shahpasandzadeh MH, Shahpasandzadeh M, Torabi M, Parirokh M. A comparative study on dental pulp response to calcium hydroxide, white and grey mineral trioxide aggregate as pulp capping agents. J Conserv Dent. 2011; 14:351–355.

5. Faraco IM Jr, Holland R. Response of the pulp of dogs to capping with mineral trioxide aggregate or a calcium hydroxide cement. Dent Traumatol. 2001; 17:163–166.

6. Accorinte ML, Loguercio AD, Reis A, Carneiro E, Grande RH, Murata SS, Holland R. Response of human dental pulp capped with MTA and calcium hydroxide powder. Oper Dent. 2008; 33:488–495.

7. Zarrabi MH, Javidi M, Jafarian AH, Joushan B. Histologic assessment of human pulp response to capping with mineral trioxide aggregate and a novel endodontic cement. J Endod. 2010; 36:1778–1781.

8. Torabinejad M, Hong CU, Pitt Ford TR, Kaiyawasam SP. Tissue reaction to implanted super-EBA and mineral trioxide aggregate in the mandible of guinea pigs: a preliminary report. J Endod. 1995; 21:569–571.

9. Moretton TR, Brown CE Jr, Legan JJ, Kafrawy AH. Tissue reactions after subcutaneous and intraosseous implantation of mineral trioxide aggregate and ethoxybenzoic acid cement. J Biomed Mater Res. 2000; 52:528–533.

10. Kettering JD, Torabinejad M. Investigation of mutagenicity of mineral trioxide aggregate and other commonly used root-end filling materials. J Endod. 1995; 21:537–542.

11. Torabinejad M, Watson TF, Pitt Ford TR. Sealing ability of a mineral trioxide aggregate when used as a root end filling material. J Endod. 1993; 19:591–595.

12. Lee SJ, Monsef M, Torabinejad M. Sealing ability of a mineral trioxide aggregate for repair of lateral root perforations. J Endod. 1993; 19:541–544.

13. Schwartz RS, Mauger M, Clement DJ, Walker WA 3rd. Mineral trioxide aggregate: a new material for endodontics. J Am Dent Assoc. 1999; 130:967–975.

14. Holland R, de Souza V, Nery MJ, Faraco Júnior IM, Bernabé PF, Otoboni Filho JA, Dezan Júnior E. Reaction of rat connective tissue to implanted dentin tube filled with mineral trioxide aggregate, Portland cement or calcium hydroxide. Braz Dent J. 2001; 12:3–8.
15. Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review-part III: clinical applications, drawbacks, and mechanism of action. J Endod. 2010; 36:400–413.

16. Jang YE, Lee BN, Koh JT, Park YJ, Joo NE, Chang HS, Hwang IN, Oh WM, Hwang YC. Cytotoxicity and physical properties of tricalcium silicate-based endodontic materials. Restor Dent Endod. 2014; 39:89–94.

17. Zanini M, Sautier JM, Berdal A, Simon S. Biodentine induces immortalized murine pulp cell differentiation into odontoblast-like cells and stimulates biomineralization. J Endod. 2012; 38:1220–1226.

18. Koubi S, Elmerini H, Koubi G, Tassery H, Camps J. Quantitative evaluation by glucose diffusion of microleakage in aged calcium silicate-based open-sandwich restorations. Int J Dent. 2012; 2012:105863.

19. Nowicka A, Lipski M, Parafiniuk M, Sporniak-Tutak K, Lichota D, Kosierkiewicz A, Kaczmarek W, Buczkowska-Radlińska J. Response of human dental pulp capped with biodentine and mineral trioxide aggregate. J Endod. 2013; 39:743–747.

20. Camilleri J, Sorrentino F, Damidot D. Investigation of the hydration and bioactivity of radiopacified tricalcium silicate cement, Biodentine and MTA Angelus. Dent Mater. 2013; 29:580–593.

21. Lee BN, Lee KN, Koh JT, Min KS, Chang HS, Hwang IN, Hwang YC, Oh WM. Effects of 3 endodontic bioactive cements on osteogenic differentiation in mesenchymal stem cells. J Endod. 2014; 40:1217–1222.

22. Jung JY, Woo SM, Lee BN, Kon JT, Nör JE, Hwang YC. Effect of Biodentine and Bioaggregate on odontoblastic differentiation via mitogen-activated protein kinase pathway in human dental pulp cells. Int Endod J. 2015; 48:177–184.

23. Zhang H, Pappen FG, Haapasalo M. Dentin enhances the antibacterial effect of mineral trioxide aggregate and bioaggregate. J Endod. 2009; 35:221–224.

24. Hashem AA, Wanees Amin SA. The effect of acidity on dislodgment resistance of mineral trioxide aggregate and bioaggregate in furcation perforations: an in vitro comparative study. J Endod. 2012; 38:245–249.

25. Grech L, Mallia B, Camilleri J. Investigation of the physical properties of tricalcium silicate cement-based root-end filling materials. Dent Mater. 2013; 29:e20–e28.

26. Batur YB, Acar G, Yalcin Y, Dindar S, Sancakli H, Erdemir U. The cytotoxic evaluation of mineral trioxide aggregate and bioaggregate in the subcutaneous connective tissue of rats. Med Oral Patol Oral Cir Bucal. 2013; 18:e745–e751.

27. Nielsen RB, Alyassin AM, Peters DD, Carnes DL, Lancaster J. Microcomputed tomography: an advanced system for detailed endodontic research. J Endod. 1995; 21:561–568.

28. Verma P, Love RM. A Micro CT study of the mesiobuccal root canal morphology of the maxillary first molar tooth. Int Endod J. 2011; 44:210–217.

29. Iwamoto CE, Adachi E, Pameijer CH, Barnes D, Romberg EE, Jefferies S. Clinical and histological evaluation of white ProRoot MTA in direct pulp capping. Am J Dent. 2006; 19:85–90.
30. Aeinehchi M, Eslami B, Ghanbariha M, Saffar AS. Mineral trioxide aggregate (MTA) and calcium hydroxide as pulp-capping agents in human teeth: a preliminary report. Int Endod J. 2003; 36:225–231.

31. Nair PN, Duncan HF, Pitt Ford TR, Luder HU. Histological, ultrastructural and quantitative investigations on the response of healthy human pulps to experimental capping with mineral trioxide aggregate: a randomized controlled trial. Int Endod J. 2008; 41:128–150.

32. Koh ET, McDonald F, Pitt Ford TR, Torabinejad M. Cellular response to mineral trioxide aggregate. J Endod. 1998; 24:543–547.

33. Seux D, Couble ML, Hartmann DJ, Gauthier JP, Magloire H. Odontoblast-like cytodifferentiation of human dental pulp cells
in vitro in the presence of a calcium hydroxide-containing cement. Arch Oral Biol. 1991; 36:117–128.

34. Laurent P, Camps J, About I. Biodentine(TM) induces TGF-β1 release from human pulp cells and early dental pulp mineralization. Int Endod J. 2012; 45:439–448.

35. Laurent P, Camps J, De Méo M, Déjou J, About I. Induction of specific cell responses to a Ca
3SiO
5-based posterior restorative material. Dent Mater. 2008; 24:1486–1494.

36. Tran XV, Gorin C, Willig C, Baroukh B, Pellat B, Decup F, Opsahl Vital S, Chaussain C, Boukpessi T. Effect of a calcium-silicate-based restorative cement on pulp repair. J Dent Res. 2012; 91:1166–1171.

37. Yan P, Yuan Z, Jiang H, Peng B, Bian Z. Effect of bioaggregate on differentiation of human periodontal ligament fibroblasts. Int Endod J. 2010; 43:1116–1121.

38. Yuan Z, Peng B, Jiang H, Bian Z, Yan P. Effect of bioaggregate on mineral-associated gene expression in osteoblast cells. J Endod. 2010; 36:1145–1148.

39. Zhang S, Yang X, Fan M. BioAggregate and iRoot BP Plus optimize the proliferation and mineralization ability of human dental pulp cells. Int Endod J. 2013; 46:923–929.

40. Saghiri MA, Tanideh N, Garcia-Godoy F, Lotfi M, Karamifar K, Amanat D. Subcutaneous connective tissue reactions to various endodontic biomaterials: an animal study. J Dent Res Dent Clin Dent Prospects. 2013; 7:15–21.
41. Tziafas D, Belibasakis G, Veis A, Papadimitriou S. Dentin regeneration in vital pulp therapy: design principles. Adv Dent Res. 2001; 15:96–100.
42. Min KS, Park HJ, Lee SK, Park SH, Hong CU, Kim HW, Lee HH, Kim EC. Effect of mineral trioxide aggregate on dentin bridge formation and expression of dentin sialoprotein and heme oxygenase-1 in human dental pulp. J Endod. 2008; 34:666–670.

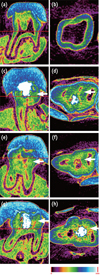
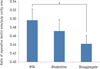
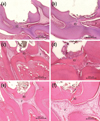
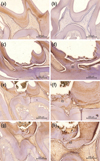




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download