Abstract
Purpose
During laparoscopic gastrectomy, an aberrant left hepatic artery (ALHA) arising from the left gastric artery (LGA) is occasionally encountered. The aim of this study was to define when an ALHA should be preserved during laparoscopic gastrectomy.
Materials and Methods
From August 2009 to December 2014, 1,340 patients with early gastric cancer underwent laparoscopic distal gastrectomy. One hundred fifty patients presented with an ALHA; of the ALHA was ligated in 116 patients and preserved in 34 patients. Patient characteristics, postoperative outcomes and perioperative liver function tests were reviewed retrospectively. Correlations between the diameter of the LGA measured on preoperative abdominal computed tomography and postoperative liver enzyme levels were analyzed.
Results
Pearson's correlation analysis showed a positive correlation between the diameter of the LGA and serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels on postoperative day 1 in the ALHA-ligated group (P=0.039, P=0.026, respectively). Linear regression analysis estimated the diameter of the LGA to be 5.1 mm and 4.9 mm when AST and ALT levels were twice the normal limit on postoperative day 1.
Anatomic variation in the hepatic artery has been reported to occur in approximately 30% of the population.123 The most common variant is an aberrant left hepatic artery (ALHA) arising from the left gastric artery (LGA). Estimates of the prevalence of ALHAs that arise from the LGA range between 6.0% and 22% of the population.124 These variants are occasionally encountered during surgery for the treatment of gastric cancer (Fig. 1). During open surgery, palpation of the lesser sac helps to identify ALHAs that arise from the LGA, and palpation of the hepatoduodenal ligament helps to identify whether this ALHA is either replaced or accessory. However, this identification is challenging considering the limited view provided in laparoscopic gastrectomy, especially in obese patients with bulky lesser sac.
Laparoscopic gastrectomy has become widespread for the treatment of early gastric cancer.5 During curative surgery for gastric cancer, the LGA should be ligated at its origin during complete lymph node dissections.6 When an ALHA is present, it should be sacrificed in order to ligate the LGA at its origin. Shinohara et al.7 reported that there was no difference in the therapeutic effects of lymph node dissection around the LGA between patients who had their ALHA preserved during surgery and those who had their ALHA ligated. Oki et al.4 have even suggested the ALHA be preserved whenever it is encountered during laparoscopic gastrectomy.
The major consequence of ALHA ligation is transient hepatic dysfunction. However, in patients with chronic liver disease, who are less tolerant to liver injury, ligation could lead to fatal liver failure.8 Indeed, lethal complications, including left hepatic lobe necrosis, after ligation of the ALHAs have been reported in the literature.910 Generally, it is thought that ligation of a large ALHA would result in postoperative hepatic dysfunction. However, the evidence for this is weak, and there is no consensus on when and in which patients the ALHA should be preserved during laparoscopic gastrectomy.
The aim of this study was to define when the ALHA should be preserved during laparoscopic gastrectomy performed during the treatment of gastric cancer.
From August 2009 to December 2014, 1,340 patients diagnosed with early gastric cancer underwent a laparoscopic distal gastrectomy at the Samsung Medical Center (Seoul, Korea). According to operation records, an ALHA was identified in 168 patients. After the exclusion of 18 patients who underwent combined operations, 150 were analyzed. The ALHA was ligated in 116 patients and preserved in 34 patients during the operation. The decision to preserve or ligate the ALHA was made by the surgeon during the surgery on a case-by-case basis.
Patient characteristics were compared between the ALHA-ligated and the ALHA-preserved groups. Preoperative abdominal computed tomography images of these patients were reviewed retrospectively, and the internal diameter of the LGA was measured (Fig. 2). Postoperative outcomes such as the operating time, estimated blood loss, length of the hospital stay after the operation, and number of lymph nodes retrieved were acquired from medical records and compared between the two groups. The operating time was measured from initiation of incision to closure of the wound. Estimated blood loss was evaluated based on anesthesia records. All patients had underwent laboratory tests for aspartate aminotransferase (AST) and alanine aminotransferase (ALT) prior to surgery and on postoperative days 1 and 5 to assess liver function
Chi-square tests and unpaired Student's t-tests were used to compare variables between the ALHA-ligated and ALHA-preserved groups. Both the median and the range were reported for continuous variables. A generalized estimating equation analysis was performed to show the changing pattern in liver function tests before and after surgery. In the subgroup analysis, Pearson correlation analysis and linear regression were performed to assess the relationships between the LGA diameter and variables extracted from postoperative laboratory data. All statistical analyses were executed using SAS ver. 9.4 software (SAS Institute Inc., Cary, NC, USA), and a P-value of <0.05 was considered statistically significant.
Of the 1,340 patients that underwent laparoscopic distal gastrectomy, 168 patients (12.5%) had an ALHA. Patient characteristics are shown in Table 1. The gender and median age of the patients were not significantly different between the ALHA-ligated and ALHA-preserved groups. The body mass index of the ALHA ligated group was significantly higher than that of eth preserved group (P=0.027). There were 6 patients with chronic hepatitis B in the ALHA-ligated group; none of the patients in the ALHA-preserved group had liver disease. More patients underwent Billroth I anastomosis in the ALHA-preserved group. The T and N stage status of the patients did not show a difference between the groups (P=0.313, P=0.212).
The operative outcomes of the groups are shown in Table 2. The median operation time was 151.5 minutes (range, 84~315 minutes) and 177.5 minutes (range, 118~329 minutes) for the ALHA-ligated group and ALHA-preserved groups, respectively, and this difference was not statistically significant. The median estimated blood loss was 100 ml in both groups and the median hospital stay after the operation was the same for both groups. The median number of lymph nodes retrieved was 37 cases (range, 16~87 cases) in the ALHA-ligated group and 33 cases (range, 16~66 cases) in the ALHA-preserved group; this difference was not statistically significant (P=0.207). The median diameter of the LGA was 4.6 mm in the ALHA-ligated group (range, 3.1~5.6 mm) and 5.1 mm in the ALHA-preserved group (range, 4.4~5.9 mm), which was a statistically significant difference (P=0.000).
Perioperative sequential changes in liver enzymes are presented in Table 2 and Fig. 3. The median serum levels of AST and ALT on postoperative day 1 were significantly higher in the ALHA-ligated group (32 IU/L [14~770 IU/L] and 37.5 IU/L [9~728 IU/L]) than in the ALHA-preserved group (27.5 IU/L [16~120 IU/L] and 29.5 IU/L [11~128 IU/L]) (P=0.009, P=0.003, respectively). Furthermore, serum levels of ALT on postoperative day 5 were significantly higher in the ALHA-ligated group (21 IU/L [6~387 IU/L]) than in the ALHA-preserved group (17 IU/L [6~54 IU/L]), P=0.007. Serum AST levels on postoperative day 5 were not statistically different between the groups.
Both groups showed elevated AST and ALT levels on postoperative day 1, with a gradual decrease back to the preoperative levels (Fig. 3). Generalized estimating equation analysis revealed statistically significant differences between the groups in terms of AST levels on postoperative day1 (P=0.014) and ALT levels on postoperative days 1 (P=0.009) and day 5 (P=0.030).
Upon subgroup analysis of the ALHA-ligated group, the diameter of the LGA and serum AST and ALT levels on postoperative day 1 were positively correlated (Pearson correlation analysis, P=0.039 and P=0.026, respectively; Fig. 4). Linear regression analysis estimated the diameter of the LGA to be 5.1 mm and 4.9 mm when the postoperative day 1 AST and ALT concentration was 80 IU/L, twice the upper limit of normal.
During laparoscopic gastrectomy, after dividing the right gastric artery, an ALHA can be easily visualized after retracting the liver and pulling the stomach down caudally in the lesser sac (Fig. 1). However, for obese patients, identification of an ALHA inside bulky soft tissue remains a challenge. Iatrogenic injuries to the ALHA and inevitable ligation of the ALHA can occur. Our study results showed that the ALHA-ligated group had a significantly higher body mass index than the ALHA-preserved group (Table 1, P=0.027). Obese patients with an injured ALHA may have been included in the ALHA-ligated group.
Surgical techniques concerning preservation of an ALHA during laparoscopic gastrectomy have been introduced by Oki et al.4 Our results did not show significant differences between the two groups in terms of operation time, estimated blood loss, the number of lymph nodes retrieved, or the length of the hospital stay after surgery (Table 2). These findings are concordant with previous studies that support the feasibility of preserving ALHAs with the same oncologic outcome.478
An ALHA can be classified as either a replaced artery, which is a substitute for a normal left hepatic artery, or an accessory artery, which is an addition to the normal left hepatic artery.47 This classification is difficult for surgeons, especially as laparoscopic approaches limit tactile sense. Previous studies have suggested conducting preoperative evaluation using three-dimensional computed tomography (CT) angiography before gastrectomy to assess vascular anatomy.111213 However, the detection rate of ALHAs was lower than the incidence previously reported,12 which may represent difficulty in identifying ALHAs using angiography.14 Differentiating between replaced and accessary ALHAs would be even more difficult using angiography. Replaced ALHAs should obviously be preserved to avoid postoperative hepatic dysfunction. Studies have shown that some accessory ALHAs are the only supplying vessel to a specific territory of the liver, which suggests that they should be preserved same as replacing arteries.15 Therefore, ligation of either replaced or accessory ALHAs could result in postoperative hepatic dysfunction.
In this study, we found transient elevation in the level of liver enzymes after ligation of the ALHA, which is concordant with previous studies.7816 Although we did not find any lethal complications, such as hepatic necrosis, we did experienced extreme elevation in the level of liver enzymes (i.e., more than 10 times higher than the normal upper limit). All patients who had elevated liver enzymes also displayed a gradual decrease, back to preoperative levels at the time of discharge with supportive care. This phenomenon could be explained by the formation of collateral arterial flow after hepatic artery ligation, as has been demonstrated by previous studies.1718
Generally, it is thought that ligation of a large ALHA could result in postoperative elevation of liver enzymes. Surgeons may have a tendency to preserve large ALHA. In our study, patients who had an ALHA preserved had thicker LGAs than those who had their ALHA ligated. This might suggest that a large ALHA would arise from a large LGA. We hypothesized that a large ALHA would arise from the large LGA, which could reflect postoperative liver dysfunction after ligation of the ALHA. We chose to measure the diameter of the LGA instead of the ALHA because identifying the ALHA was difficult upon examining preoperative CT images. We also wanted to find an indicator that could help to identify ALHAs during laparoscopic gastrectomy. When surgeons encounter large size LGAs during surgery, they should review the preoperative CT scan to check the luminal diameter of the LGA. During laparoscopic gastrectomy, the diameter of the LGA could be estimated by comparing it with the thickness of laparoscopic instruments.
We found that the diameter of the LGA correlated positively with elevated serum levels of AST and ALT on postoperative day 1 in the ALHA-ligated group. This result supports our hypothesis that ligation of an ALHA arising from a large LGA could result in postoperative liver dysfunction. Based on the linear regression analysis we performed, an ALHA rising from an LGA larger than 5 mm in diameter should be preserved to avoid postoperative elevation of liver enzymes. Therefore, surgeons encountering a large LGA during laparoscopic gastrectomy should carefully examine the lesser sac for the presence of an ALHA before ligating the LGA at its origin. Especially in obese patients, for whom special attention is needed when dissecting the lesser sac.
Fortunately in our study, patients with chronic hepatitis B in ALHA ligated group had LGAs smaller than 5 mm in diameter, and these patient had postoperative AST/ALT levels increased less than twice the normal range. Further study is needed whether to determine whether to apply the same standard in patients with liver disease.
Preservation of an ALHA during laparoscopic gastrectomy is feasible. Surgeons should be aware of ALHA when dissecting the lesser sac during laparoscopic gastrectomy. We suggest preserving ALHA, arising from a large LGA, diameter larger than 5 mm, during laparoscopic gastrectomy to prevent immediate postoperative hepatic dysfunction. Our suggestion may not only be helpful in laparoscopic gastrectomy, but also in other upper abdominal surgery, such as bariatric surgery. As this study is a retrospective clinical analysis, confirmation with further prospective study in a large number of patients will be needed in the future.
Figures and Tables
Fig. 1
Photographs from the laparoscopic view during laparoscopic gastrectomy. (A) Identifying the aberrant left hepatic artery (ALHA) in the gastrohepatic ligament. (B) Skeletonization of the aberrant left hepatic artery arising from the left gastric artery (LGA). (C) Photograph after preserving the ALHA from the LGA. Splenic a. = splenic artery.

Fig. 2
Image of the abdominal computed tomography measuring the diameter of the left gastric artery. The white arrow differentiates between the the aberrant left hepatic artery and the left gastric artery.
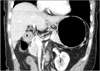
Fig. 3
Sequential changes in postoperative liver enzymes. Each value is the mean. Preop = preoperative; POD = postoperative day. *P<0.05.

Fig. 4
Scatter diagram showing a positive correlation between the diameter of the left gastric artery (LGA) and postoperative levels of liver enzymes. POD = postoperative day; AST = aspartate aminotransferase; ALT = alanine aminotransferase.

References
1. Michels NA. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg. 1966; 112:337–347.
2. Hiatt JR, Gabbay J, Busuttil RW. Surgical anatomy of the hepatic arteries in 1000 cases. Ann Surg. 1994; 220:50–52.
3. López-Andújar R, Moya A, Montalvá E, Berenguer M, De Juan M, San Juan F, et al. Lessons learned from anatomic variants of the hepatic artery in 1,081 transplanted livers. Liver Transpl. 2007; 13:1401–1404.
4. Oki E, Sakaguchi Y, Hiroshige S, Kusumoto T, Kakeji Y, Maehara Y. Preservation of an aberrant hepatic artery arising from the left gastric artery during laparoscopic gastrectomy for gastric cancer. J Am Coll Surg. 2011; 212:e25–e27.
5. Kim YW, Yoon HM, Eom BW, Park JY. History of minimally invasive surgery for gastric cancer in Korea. J Gastric Cancer. 2012; 12:13–17.
6. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011; 14:101–112.
7. Shinohara T, Ohyama S, Muto T, Yanaga K, Yamaguchi T. The significance of the aberrant left hepatic artery arising from the left gastric artery at curative gastrectomy for gastric cancer. Eur J Surg Oncol. 2007; 33:967–971.
8. Huang CM, Chen QY, Lin JX, Zheng CH, Li P, Xie JW, et al. Short-term clinical implications of the accessory left hepatic artery in patients undergoing radical gastrectomy for gastric cancer. PLoS One. 2013; 8:e64300.
9. Lurie AS. The significance of the variant left accessory hepatic artery in surgery for proximal gastric cancer. Arch Surg. 1987; 122:725–728.
10. Hemming AW, Finley RJ, Evans KG, Nelems B, Fradet G. Esophagogastrectomy and the variant left hepatic artery. Ann Thorac Surg. 1992; 54:166–168.
11. Matsuki M, Kani H, Tatsugami F, Yoshikawa S, Narabayashi I, Lee SW, et al. Preoperative assessment of vascular anatomy around the stomach by 3D imaging using MDCT before laparoscopy-assisted gastrectomy. AJR Am J Roentgenol. 2004; 183:145–151.
12. Lee SW, Shinohara H, Matsuki M, Okuda J, Nomura E, Mabuchi H, et al. Preoperative simulation of vascular anatomy by three-dimensional computed tomography imaging in laparoscopic gastric cancer surgery. J Am Coll Surg. 2003; 197:927–936.
13. Yamashita K, Sakuramoto S, Mieno H, Shibata T, Nemoto M, Katada N, et al. Preoperative dual-phase 3D CT angiography assessment of the right hepatic artery before gastrectomy. Surg Today. 2014; 44:1912–1919.
14. Koops A, Wojciechowski B, Broering DC, Adam G, Krupski-Berdien G. Anatomic variations of the hepatic arteries in 604 selective celiac and superior mesenteric angiographies. Surg Radiol Anat. 2004; 26:239–244.
15. Miyaki T, Sakagami S, Ito H. Intrahepatic territory of the accessory hepatic artery in the human. Acta Anat (Basel). 1989; 136:34–37.
16. Okano S, Sawai K, Taniguchi H, Takahashi T. Aberrant left hepatic artery arising from the left gastric artery and liver function after radical gastrectomy for gastric cancer. World J Surg. 1993; 17:70–73. discussion 74.
17. Koehler RE, Korobkin M, Lewis F. Arteriographic demonstration of collateral arterial supply to the liver after hepatic artery ligation. Radiology. 1975; 117:49–54.
18. Mays ET, Wheeler CS. Demonstration of collateral arterial flow after interruption of hepatic arteries in man. N Engl J Med. 1974; 290:993–996.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download