Abstract
Purpose
We evaluated the clinical outcomes of the non-operative management of post-gastrectomy duodenal stump leakage in patients with gastric cancer.
Materials and Methods
A total of 1,230 patients underwent gastrectomy at our institution between 2010 and 2014. Duodenal stump leakage was diagnosed in 19 patients (1.5%), and these patients were included in this study. The management options varied with patient condition; patients were managed conservatively, with a pigtail catheter drain, or by tube duodenostomy via a Foley catheter. The patients' clinical outcomes were analyzed.
Results
Duodenal stump leakage was diagnosed in all 19 patients within a median of 10 days (range, 1~20 days). The conservative group comprised of 5 patients (26.3%), the pigtail catheter group of 11 patients (57.9%), and the Foley catheter group of 3 patients (15.8%). All 3 management modalities were successful; none of the patients needed further operative intervention. The median hospital stay was 18, 33, and 42 days, respectively.
Despite an overall decline in the incidence of gastric carcinoma, it remains the second leading cause of cancer-related deaths worldwide, with the highest prevalence in Korea.1 A major complication of gastric carcinoma is post-gastrectomy duodenal stump leakage, which can lead to intra-abdominal abscess, sepsis, and even death.234 The reported prevalence of duodenal stump leakage ranges between 1% and 6%; the mortality rate ranges between 3% and 5%, despite the development of improved surgical techniques, antibiotic therapy, and nutritional support.5 Early detection of leakage is crucial in an effort to minimize mortality and morbidity. Early diagnosis of duodenal stump leakage is all the more important given that early manifestations can include only mild and nonspecific symptoms or signs.6
Among the traditional treatment options, surgical correction with primary closure of the leaking duodenum is a difficult and sometimes ineffective procedure, due to patient instability, presence of severe edema, friable tissues, and dense adhesions in the postoperative period.7 Moreover, the possible consequences of inadequate repair–delayed leakage, widespread abdominal contamination, sepsis, death–can be devastating. An alternative treatment option is simple percutaneous drainage.8 Percutaneous tube duodenostomy has been recently introduced as an effective and safe technique for the management of continuous duodenal stump leakage.9 Tube decompression of the duodenum can initially be utilized in the management of the post-gastrectomy duodenal stump to prevent blow-out at the suture or stapler line. Thereafter, the leakage site will usually close spontaneously within 2 to 6 weeks of stoma formation. We anticipated that utilization of non-operative management of duodenal stump leakage would effectively close the leak and facilitate patients' early return to daily life and activities.
Despite good outcomes, tube duodenostomy is yet to gain universal acceptance or be properly utilized. Most of the existing studies relating to management of duodenal stump leakage are limited to a small number of case series. In this study, we reviewed a series of patients with gastric cancer, to gain insight into the effectiveness of tube duodenostomy and other modes of non-operative management for the treatment of duodenal stump leakage.
Between January 2010 and December 2014, a total of 1,230 patients underwent total or subtotal gastrectomy at our institution. Postoperative duodenal stump leakage was diagnosed in 19 patients (1.5%). Diagnosis was confirmed by postoperative abdominal computed tomography (CT) scan, which showed varied amounts of fluid collection or well-formed abscesses in the right subhepatic space or duodenal stump.
Approval for this retrospective study was obtained from the Institutional Review Board of the Seoul St. Mary's Hospital.
Duodenal stump leakage was diagnosed within the first 20 postoperative days. Upon confirmation of the diagnosis, a fasting regimen, along with antibiotics and parenteral nutrition, was initiated. The appropriate treatment methods were determined according to each patient's clinical situation. Surgery for prevention of further leakage was considered in cases of diffuse peritonitis, intra-abdominal hemorrhage, and major wound disruption. Of the 19 patients discussed, 5 patients (26.3%) were treated conservatively (i.e., with intravenous antibiotics), 11 patients (57.9%) with percutaneous drainage by pigtail catheter, and 3 patients (15.8%) by fluoroscopy-guided duodenostomy using a Foley catheter.
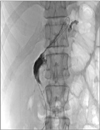
Percutaneous drainage or duodenostomy was performed as outlined in our previous report.3 Briefly, patients were scheduled for drainage catheter insertion immediately after confirmation of duodenal stump leakage (Fig. 1). Following tubography, the fluid cavity was percutaneously punctured using an 18-gauge Chiba needle (TSK Laboratory, Tochigi, Japan) under ultrasound- or cone-beam CT guidance. A pigtail catheter (Sungwon Medical, Gwangju, Korea) was inserted over a guidewire under fluoroscopic guidance (Fig. 2). Follow-up tubography was performed 1 to 3 weeks later, once the patient's acute symptoms had subsided and the discharge through the drainage catheter had either decreased or stopped.
In cases requiring a duodenostomy tube, a Foley catheter was inserted to close the fistula tract. Specifically, with the patient in the supine position, a 0.035-inch guidewire was inserted, and the pigtail catheter was removed under fluoroscopic guidance. The duodenal stump opening was probed using various types of 5F angiographic catheters, and the guidewire and catheter were inserted into the duodenal lumen via the fistula tract (Fig. 3). Finally, a Foley catheter (size range, 8~16 French) was inserted. The catheter was affixed to the skin by application of mild tension. The Foley catheter was subsequently clamped if discharge decreased for more than 2 days. If the fluid accumulation was observed to decrease on follow-up CT, and if no new symptoms or pericatheter leakage was present, the Foley catheter was removed.
Of the 19 patients in this study, majority was male patients (n=17 or 89.5%), and the mean age was 64 years (range, 28~90 years). All patients complained of high fever and showed signs of peritoneal irritation; hence, duodenal stump leakage was suspected. Six patients (31.6%) underwent initial surgery laparoscopically, while the remaining 13 patients (68.4%) underwent open-approach gastrectomy (Table 1). Almost all of the patients (94.7%) underwent distal gastrectomy; only 1 patient underwent a total gastrectomy (because the tumor location was proximal). Surgical complications, such as pulmonary issues, deep venous thrombosis, and wound infections, were encountered.
The duration of the oral food and fluid restriction varied according to the patients' respective clinical parameters; the mean fasting period was 10 days. The patients in the Foley catheter group were able to resume oral intake much earlier (9.3 days for conservative vs. 3.0 days for Foley catheter vs. 10.5 days for pigtail).
One of the 3 patients in the Foley catheter group experienced a recurrence of symptoms 10 days after catheter removal and subsequent discharge from the hospital. A follow-up CT showed a recurrent loculated fluid cavity in the subhepatic space, which was highly suggestive of persistent stump leakage. For this patient, a Foley catheter was again inserted and removed 2 months later, without further complication.
The value of duodenal tube decompression for post-gastrectomy management of the duodenal stump was demonstrated for the first time in 1954.10 Over the many decades thereafter, tube duodenostomy has been a successful method for management of difficult duodenal stump leakage; this notwithstanding, it has still not gained widespread acceptance. Post-repair complex duodenal injury leakage is more common than post-gastrectomy duodenal stump leakage. Although most duodenal perforations can be effectively managed using simple repairs, more complex injuries require complicated procedures.
Surgery should be considered in cases of diffuse peritonitis, intra-abdominal hemorrhage, or major wound disruption to prevent further leakage and to promote early maturation of the fistula tract.11 The patient's overall clinical situation, the availability of clinical facilities, and the presence or absence of peritonitis are all major factors determining the best management pathway. Many surgical options for the treatment of the 'difficult-to-manage' duodenum have been explored. With regard to the present series, 5 reoperations for duodenal stump leakage (Table 2) were necessary: 1 was due to disseminated peritonitis, 2 for bleeding, and another 2 for intestinal obstruction. All 19 cases needed drainage procedures, and in 2 of them, duodenostomy was performed.
Tube duodenostomy, in contrast to surgical intervention, is a simple technique, does not involve general anesthesia, and is easy to learn, teach, and perform.12 Several early papers indicate no changes in either outcome or leakage rate for this method,13 which probably has contributed to its current lack of acceptance. A more recent study however, in contrast to the data presented in those early papers, demonstrates excellent outcomes with no leakage recurrence.11 We believe that tube duodeneostomy, by obviating the need for complex surgical intervention and the concomitant increased morbidity and extended hospitalization, provides an opportunity to stabilize the patient, thus allowing future surgery where the possibility for transfer exists or where subspecialty expertise might be required.
Current management of the perforated duodenal stump leaves much to be desired. Even with prompt diagnosis and aggressive treatment, the levels of morbidity and mortality remain high. It is generally accepted that suture closure of the perforated duodenal stump is inadvisable, as it can lead to edema and adhesions. Treatment, therefore, has been directed towards drainage of the duodenal lumen and extraduodenal areas. Supplementary nutritional support and electrolyte replacement intravenously or via feeding jejunostomy, furthermore, should be initiated as early as possible. If complications are prevented by early closure of the perforated stump (thereby removing the source of contamination), mortality and morbidity rates can be markedly lowered.
Unfortunately, this method is not without complications. Technical failure or an uncontrolled source of sepsis, for example, would necessitate further intervention. Among the undesirable after-effects of catheter duodenostomy, meanwhile, are persistent duodeno-cutaneous fistula and prolonged sinus drainage. Moreover, under certain circumstances, such conditions, if sufficiently prolonged, can necessitate re-operation. Erosion of duodenal tissues by rubber catheters and subsequent hemorrhaging have also been observed, though in most cases, bleeding stops spontaneously. Yet another identified complication of catheter duodenostomy is leakage of duodenal content around the tube or displacement of the tube from the duodenum, with resulting peritonitis. Deliberate formation of a duodenal fistula necessarily entails acceptance of certain potential problems concerning fluid and electrolyte management. The average post-gastrectomy hospitalization period in the present series managed by catheter duodenostomy was 18.1 days. An understanding of the current status of the 'difficult to manage' duodenal stump, as noted above, was instrumental in stimulating our interest in the development of an alternative approach to this vexing problem.
This study has various limitations. It is retrospective in design, with a short-term follow-up period and a small sample size. In addition, the pigtail catheter indwelling and Foley-catheter placement times varied among the cases. Despite these limitations, our results showed that postoperative duodenal stump leakage, an otherwise very problematic issue, can be treated effectively and safely using a pigtail or Foley catheter. In particular, the main advantages of this procedure are its avoidance of unnecessary aggressive intervention, promotion of early oral intake, and permittance of early hospital discharge.
In conclusion, fluoroscopy-guided percutaneous catheter placement can be considered a safe and effective treatment option for post-gastrectomy duodenal stump leakage in patients with gastric cancer.
Figures and Tables
Fig. 1
Abdominal computed tomography scan showing duodenal stump leakage and air pockets suggestive of abscess formation.
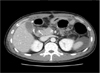
Table 1
Detailed patient data and procedural results

pTNM = pathological tumor-node metastasis; AJCC, American Joint Committee on Cancer; POD = postoperative day; NPO = null per os; M = male; F = female; Lap = laparoscopic; con. = converted; DG = distal gastrectomy; TG = total gastrectomy; B2 = billroth II reconstruction; R-Y = Roux-en-Y; D = day; DVT = deep vein thrombosis; CVA = cerebrovascular accident; HTN = hypertension; DM = diabetes mellitus; IHD = ischemic heart disease; BA = bronchial asthma.
References
1. Strong VE, Song KY, Park CH, Jacks LM, Gonen M, Shah M, et al. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann Surg. 2010; 251:640–646.
2. Golub R, Golub RW, Cantu R Jr, Stein HD. A multivariate analysis of factors contributing to leakage of intestinal anastomoses. J Am Coll Surg. 1997; 184:364–372.
3. Hur H, Lim YS, Jeon HM, Kim W. Management of anastomotic leakage after gastrointestinal surgery using fluoroscopy-guided foley catheter. J Korean Surg Soc. 2010; 78:165–170.
4. Li J, Ren J, Zhu W, Yin L, Han J. Management of enterocutaneous fistulas: 30-year clinical experience. Chin Med J (Engl). 2003; 116:171–175.
5. Tøttrup A. Foley catheter enterostomy for postoperative bowel perforation: an effective source control. World J Surg. 2010; 34:2752–2754.
6. Yoo HM, Lee HH, Shim JH, Jeon HM, Park CH, Song KY. Negative impact of leakage on survival of patients undergoing curative resection for advanced gastric cancer. J Surg Oncol. 2011; 104:734–740.
7. Pickleman J, Watson W, Cunningham J, Fisher SG, Gamelli R. The failed gastrointestinal anastomosis: an inevitable catastrophe? J Am Coll Surg. 1999; 188:473–482.
8. Papanicolaou N, Mueller PR, Ferrucci JT Jr, Dawson SL, Johnson RD, Simeone JF, et al. Abscess-fistula association: radiologic recognition and percutaneous management. AJR Am J Roentgenol. 1984; 143:811–815.
9. Oh JS, Lee HG, Chun HJ, Choi BG, Lee SH, Hahn ST, et al. Percutaneous management of postoperative duodenal stump leakage with foley catheter. Cardiovasc Intervent Radiol. 2013; 36:1344–1349.
10. Welch CE, Rodkey GV. A method of management of the duodenal stump after gastrectomy. Surg Gynecol Obstet. 1954; 98:376–379.
11. Aurello P, Sirimarco D, Magistri P, Petrucciani N, Berardi G, Amato S, et al. Management of duodenal stump fistula after gastrectomy for gastric cancer: systematic review. World J Gastroenterol. 2015; 21:7571–7576.
12. Isik B, Yilmaz S, Kirimlioglu V, Sogutlu G, Yilmaz M, Katz D. A life-saving but inadequately discussed procedure: tube duodenostomy. Known and unknown aspects. World J Surg. 2007; 31:1616–1624.
13. Burch JM, Cox CL, Feliciano DV, Richardson RJ, Martin RR. Management of the difficult duodenal stump. Am J Surg. 1991; 162:522–526.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download