Abstract
Gastric metastasis from ovarian carcinoma is extremely rare and the prognosis for patients is poor. We report a case of multimodal treatment improving the survival time of a patient with gastric metastasis from ovarian cancer. A 73-year-old woman with known serous ovarian cancer was admitted to the hospital due to epigastric pain and dyspepsia. On esophagogastroduodenoscopy, a protruding mass was noted at the gastric antrum. She underwent distal gastrectomy with Billroth I anastomosis and lymph node dissection, including the para-aortic lymph nodes. The final pathology revealed gastric metastasis from ovarian serous adenocarcinoma. In this case, after cytoreductive surgery, chemotherapy was performed each time a recurrence was diagnosed, and remission was accomplished. She survived for 108 months after the first diagnosis of the metastatic tumor in the stomach. Multimodal treatment of metastatic lesions since the first diagnosis allowed the patient to survive longer than those in previous reports.
The stomach is an unusual site for metastasis. Most gastric metastases are reported to arise from breast cancer, lung cancer, or melanoma.12345 Gastric metastasis from ovarian carcinoma is extremely rare because ovarian carcinoma usually metastasizes along the peritoneal surface. The presence of gastric metastasis is likely to be a preterminal event, and therefore the prognosis for patients is poor and the survival period is extremely short. Here, we report a case of multimodal treatment improving the survival of a patient with gastric metastasis from ovarian cancer.
In March 2004, a 73-year-old woman presented to our hospital with a 3-week history of epigastric pain and dyspepsia. She had no hematemesis, melena, weight loss, or any other clinical manifestations.
Seven years previously, she had undergone cytoreductive surgery in our hospital for ovarian serous adenocarcinoma, followed by six cycles of adjuvant chemotherapy with paclitaxel and carboplatin. In July 1999, when a right adnexal mass was found on pelvic computed tomography (CT), she underwent secondary debulking cytoreductive surgery, followed by six cycles of adjuvant chemotherapy with paclitaxel and carboplatin. In March 2000, the patient underwent third-look laparotomy that revealed no evidence of intra-abdominal disease.
In November 2000, a 4×3-cm cystic mass in the rectum was found on a CT scan, and she underwent low anterior resection with multiple metastatectomy, including partial vaginectomy. Five cycles of adjuvant chemotherapy with topotecan were administered. In November 2001, a CT scan detected multiple enlarged para-aortic lymph nodes, and a punch biopsy of the vaginal stump showed a recurrent adenocarcinoma. She received another six cycles of chemotherapy with docetaxel and carboplatin, followed by whole pelvic radiotherapy (a total dose of 39.6 Gy in 22 fractions) with vaginal vault brachytherapy (a dose of 20 Gy in five fractions).
The patient remained free of disease until March 2004, when a CT scan detected a 6.5×6.0-cm mass, compressing the gastric antrum and body, suggestive of a metastatic node of the omentum. The scan also showed perihepatic, perigastric, and para-aortic lymph node involvement (Fig. 1). On esophagogastroduodenoscopy, a protruding mass was noted in the gastric antrum. The biopsies revealed adenocarcinoma of unknown origin.
The patient underwent explorative laparotomy. A 7×5-cm isolated mass on the greater curvature of the stomach and multiple enlarged perigastric, perihepatic, celiac, and para-aortic lymph nodes were found. A distal gastrectomy with a Billroth I anastomosis and lymph node dissection, including the para-aortic lymph nodes, was performed.
The resected stomach had a protruding tumor that measured 7×5-cm with central ulceration (Fig. 2A). On a cut section, this white-yellow tumor was situated in the muscularis propria, and bulged into the serosa (Fig. 2B). The histopathologic findings revealed a gastric metastasis from the ovarian serous adenocarcinoma. The patient underwent six cycles of adjuvant chemotherapy with cisplatin and cyclophosphamide.
Two years after the gastric surgery, hypermetabolic lesions in the liver and spleen were observed on 18F-fluorodeoxyglucose positron emission tomography with computed tomography (FDG-PET/CT). Partial segmentectomy of the liver, splenectomy, and partial excision of the diaphragm were performed. In July 2007 and August 2008, FDG-PET/CT showed aortocaval and para-aortic lymph node metastases. Six cycles of chemotherapy with gemcitabine and cisplatin were administered along with para-aortic radiotherapy (a total dose of 45 Gy in 25 fractions) was given. Follow-up remained negative until July 2010, when a hypermetabolic mass in the remnant stomach was observed on FDG-PET/CT.
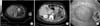
In March 2011, the size of the hypermetabolic mass increased (Fig. 3A). An enhanced abdominal CT demonstrated recurrent gastric cancer with invasion to the pancreas (Fig. 3B). On esophagogastroduodenoscopy, a normal mucosal covered mass was noted at the proximal site of the anastomosis. Biopsies revealed chronic gastritis with erosion (Fig. 3C).
The patient received chemotherapy with capecitabine for 7 months, but the disease progressed to massive intrahepatic metastasis. Finally, she was referred to hospice care and died 3 months later.
Gastric metastasis from ovarian carcinoma is extremely rare.1234567 Ovarian carcinoma usually metastasizes to peritoneal surfaces by exfoliating cells that implant throughout the peritoneum, and the intraperitoneal route of dissemination is the most common mode of dissemination. Gastrointestinal involvement is usually limited to the seromuscular layer of the bowel and its mesentery. In this case, the patient experienced recurrences by the intraperitoneal route of dissemination in 1999, 2000, and 2001. However, gastric metastasis also occurs via lymphatic channels or through the hematogenous route. Because the stomach receives a rich blood supply, it should be considered as a possible target organ of metastasis. The absence of peritoneal seeding and involvement of the entire gastric wall suggest blood-born metastasis in this case.
Clinical manifestations of metastasis to the stomach are non-specific and include epigastric pain, melena, anemia, nausea, and vomiting. Solitary metastasis is more common than multiple metastases, and the metastases are more frequently located in the middle or upper third of the stomach. Although the endoscopic appearance often resembles that of a submucosal tumor or primary gastric cancer, the final diagnosis is easily obtained in over 90% of cases from endoscopic biopsies.124567
Cytoreductive surgery to minimize residual tumor followed by platinum- and taxane-based chemotherapy is the standard treatment for advanced epithelial ovarian cancer. In this case, after cytoreductive surgery, chemotherapy was performed each time a recurrence was diagnosed, and remission was accomplished. Additional systemic chemotherapy was administered when another recurrence was diagnosed in the stomach. The treatment for metastatic tumors in the stomach usually consists of systemic therapy rather than surgery. However, when there is a risk of bleeding, tumor perforation, an uncertain diagnosis, or a solitary metastasis, surgical resection may be recommended to control hemorrhaging, thus improving the patient's quality of life.
In this case, metastases in the liver and spleen were found 2 years after gastric surgery. It was uncertain whether these metastases originated from the metastatic tumor (in the stomach), or from the primary tumor (in the ovary). Hepatic parenchymal metastases of ovarian cancer can be divided into two groups: 1) unresectable, hematogenous hepatic parenchymal metastasis and 2) resectable, hepatic parenchymal metastasis from peritoneal seeding. Most hepatic parenchymal metastases come from peritoneal seeding. In some patients, hepatic metastases are present at the time of diagnosis (synchronous metastases), but more frequently, they represent the evolution of the disease after surgery (metachronous metastases).8 Metastatic carcinoma to the spleen is rare. In most cases, the spleen is involved as part of a diffuse carcinomatosis and splenic metastasis reflects widespread hematogenous tumor dissemination.
Because the presence of gastric metastasis is likely to be a preterminal event, the prognosis for patients is poor, and the survival period is extremely short. The median interval between the treatment of the primary tumor and the diagnosis of a metastatic tumor in the stomach has been reported to be 16 to 78 months for a variety of primary cancers.2 Kobayashi et al.9 reported that the median survival time after a metastatic gastric tumor diagnosis was 170 days (range, 16~892 days) for all cases; 384 days for those who underwent gastrectomy, and 27 days for those without active treatment. In this case, the interval between primary tumor treatment and diagnosis of the gastric metastasis was 86 months, and the patient survived for 108 months after diagnosis of the metastatic tumor in the stomach. To our knowledge, this is the longest survival time reported after a diagnosis of a metastatic gastric tumor from ovarian cancer. Multimodal treatment of metastatic lesions since the first diagnosis allowed the patient to survive longer than those in previous reports, which made this case different from other cases. In this very rare case, an ovarian serous adenocarcinoma had metastasized to multiple organs, including the stomach, liver, and spleen.
Figures and Tables
Fig. 1
Computed tomography scan of the metastatic tumor. The computed tomography scan shows a 6.5×6.0-cm mass compressing the gastric antrum and body, suggestive of a metastatic node of the omentum.
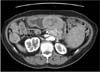
Fig. 2
Gross photograph of the stomach mass. (A) The resected stomach has a protruding tumor that measures 7×5-cm with central ulceration. (B) On the cut section, this white-yellow tumor is situated in the muscularis propria, and bulges into the serosa.
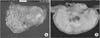
Fig. 3
Recurrence in the remnant stomach. (A) 18F-fluorodeoxyglucose positron emission tomography with computed tomography shows a hypermetabolic mass in the remnant stomach. (B) An enhanced abdominal computed tomography demonstrates recurrent gastric cancer with invasion to the pancreas. (C) On esophagogastroduodenoscopy, a normal mucosal covered mass is visible at the proximal site of the anastomosis.
References
1. De Palma GD, Masone S, Rega M, Simeoli I, Donisi M, Addeo P, et al. Metastatic tumors to the stomach: clinical and endoscopic features. World J Gastroenterol. 2006; 12:7326–7328.
2. Namikawa T, Hanazaki K. Clinicopathological features and treatment outcomes of metastatic tumors in the stomach. Surg Today. 2014; 44:1392–1399.
3. Obeidat F, Mismar A, Shomaf M, Yousef M, Fram K. Gastric perforation secondary to metastasis from ovarian cancer: Case report. Int J Surg Case Rep. 2013; 4:541–543.
4. Oda I, Kondo H, Yamao T, Saito D, Ono H, Gotoda T, et al. Metastatic tumors to the stomach: analysis of 54 patients diagnosed at endoscopy and 347 autopsy cases. Endoscopy. 2001; 33:507–510.
5. Zhou JJ, Miao XY. Gastric metastasis from ovarian carcinoma: a case report and literature review. World J Gastroenterol. 2012; 18:6341–6344.
6. Jung HJ, Lee HY, Kim BW, Jung SM, Kim HG, Ji JS, et al. Gastric metastasis from ovarian adenocarcinoma presenting as a submucosal tumor without ulceration. Gut Liver. 2009; 3:211–214.
7. Kang WD, Kim CH, Cho MK, Kim JW, Lee JS, Ryu SY, et al. Primary epithelial ovarian carcinoma with gastric metastasis mimic gastrointestinal stromal tumor. Cancer Res Treat. 2008; 40:93–96.
8. Lim MC, Kang S, Lee KS, Han SS, Park SJ, Seo SS, et al. The clinical significance of hepatic parenchymal metastasis in patients with primary epithelial ovarian cancer. Gynecol Oncol. 2009; 112:28–34.
9. Kobayashi O, Murakami H, Yoshida T, Cho H, Yoshikawa T, Tsuburaya A, et al. Clinical diagnosis of metastatic gastric tumors: clinicopathologic findings and prognosis of nine patients in a single cancer center. World J Surg. 2004; 28:548–551.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download