Abstract
Surgeons occasionally encounter a patient with a gastric cancer invading an adjacent organ, such as the pancreas, liver, or transverse colon. Although there is no established guideline for treatment of invasive gastric cancer, combined resection with radical gastrectomy is conventionally performed for curative purposes. We recently treated a patient with a large gastric cancer invading the abdominal wall, which was initially diagnosed as a simple abdominal wall abscess. Computed tomography showed that an abscess had formed adjacent to the greater curvature of the stomach. During surgery, we made an incision on the abdominal wall to drain the abscess, and performed curative total gastrectomy with partial excision of the involved abdominal wall. The patient received intensive treatment and wound management postoperatively with no surgery-related adverse events. However, the patient could not receive adjuvant chemotherapy and expired on the 82nd postoperative day.
Anatomically, the stomach resides within the upper abdomen. The adjacent structures include the spleen, transverse colon, liver, diaphragm, pancreas, abdominal wall, adrenal gland, small intestine, and retroperitoneum. Gastric cancer invading these adjacent organs is considered T4 disease. While the treatment strategy for locally advanced gastric cancer remains controversial, patients with proven T4 disease who achieve an R0 resection have a clinically and statistically significant survival advantage over those undergoing only palliative resection.1
We recently treated a patient with a large perforating gastric cancer with invasion of the abdominal wall. Gastric perforation in this case was directed toward the abdominal wall and the initial presentation was that of soft tissue infection. To protect the peritoneal cavity from bacterial contamination, we incised the abscess pocket and drained pus before entering the peritoneal cavity.
We report on this rare but critical case to discuss the optimal treatment plan and clinical course of an advanced gastric cancer invading the abdominal wall.
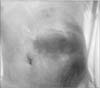
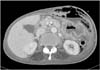
A 71-year-old woman was admitted to the emergency department of our hospital with the complaint of a painful abdominal mass. The patient was malnourished and had poor hygiene; at the time of admission, she was febrile with a temperature of 38℃ and her pulse rate was 106 beats per minute. There was no evidence of a heart murmur or lung crackles on auscultation, and the patient's breath sounds were clear. There were no signs of abdominal or rebound tenderness suggestive of peritonitis; however, there was a large tender mass in the abdominal left upper quadrant (Fig. 1). Laboratory findings included: hemoglobin, 8.3 g/dl; white blood cell count, 17,510/ml (89.3% neutrophils); blood urea nitrogen, 27.5 mg/dl; creatinine, 0.38 mg/dl; sodium, 142 mEq/L; chloride, 105 mEq/L; potassium, 3.2 mEq/L; and C-reactive protein, 304 mg/L. Urinalysis revealed no abnormalities. Chest radiography showed no abnormalities and computed tomography of the abdomen revealed a large abscess pocket on the left anterior abdonmail wall originating from the greater curvature of the stomach (Fig. 2).
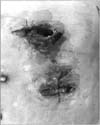
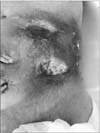
As a surgical emergency, we incised the mass and drained a large abscess. After drainage, we performed a diagnostic laparotomy. On entering the abdominal cavity through a midline incision, a huge gastric cancer invading the abdominal wall was observed, with no apparent distant metastasis. The peritoneal cavity was not contaminated due to previous abscess drainage. Dissection of the stomach from the peritoneal membrane was impossible because the stomach cancer had penetrated into the soft tissue layer of the abdominal wall (Fig. 3). Therefore, we decided to perform total gastrectomy along with resection of the involved abdominal wall, including peritoneum, rectus abdominis muscle, and subcutaneous tissue. As a result, there was no remaining muscle or subcutaneous layer on the patient's left upper quadrant, and the overlying skin was not viable due to thermal injury during the operation and a poor vascular supply (Fig. 4). There was no way to repair the defect, and only wet gauze packing of the wound was performed. We could only perform D1 lymph node dissection for the perigastric lymph nodes because the patient was hypotensive and septic. The R status was expected to be R1 for the microscopic invasion of cancer cells into the abdominal wall.
After surgery, the patient was admitted to the intensive care unit for postoperative care and received resuscitation. On the first postoperative day (POD), the vital signs became stable; however, the patient was febrile with a temperature of 38.8℃ and chest radiography showed diffuse haziness in both lung fields. With spuiscion of pneumonia, intensive treatment, including broad-spectrmu antibiotics and parenteral nutrition, was performed, and she was closely observed for suspicious leakage at the anastomosis. On the third POD, the patient resumed oral intake, and chest radiography on the seventh POD showed improvement of the pneumonia. The patient was moved to a general ward on the eighth POD and resumed ambulation. Wet dressing of the wound was continued and an abdominal binder was used to prevent evisceration of the rina-t abdominal organs through the abdominal 'hole.' We planned to perform chemotherapy after improvement in her general condition; however, the growth of the cancer progressed rapidly. On the 40th POD, the wound had filled with growing cancer (Fig. 5), and the prognosis was expected to deteriorate. After discussing treatment plans with the patient and her family, we performed conservative management, including analgesics, sedatives, and nutrition for palliation. By the 60th POD, oral intake was impossible because of her poor medical condition and intestinal obstruction. On the 82nd POD, she died of multiorgan failure caused by sepsis and pneumonia. The histologic diagnosis was poorly differentiated adenocarcinoma of the stomach. Of 13 lymph nodes harvested, 5 were cancer-cell positive (N2); the T stage could not be determined because of the anatomic disruption of the gastric serosa; however it was suspected to be T4a for cancer invasion through the gastric wall into the abdominal wall. The cancer stage was IIIB.
The management of locally advanced gastric cancer is challenging, as the balance between cancer-related mortality and operation-related complications should be considered in deciding treatment strategy. While two randomized controlled trials concluded that multiorgan resection significantly increased morbidity and mortality,23 recent retrospective studies reported that radical surgery could increase the survival rate.4 For cases with gastric cancer perforation and invasion into adjacent organs, such as this case, decision-making might be difficult, as the patient is often septic; therefore, treatment is usually straightforward life-saving salvage. Moreover, associated peritonitis and advanced cancer stage might impede curative resection. Previously, local surgery, such as simple closure of the malignant perforation, has been the preferred treatment;5 however, Heimlich6 reported in 1963 that immediate gastrectomy could improve survival. To the best of our knowledge, there has been no prospective study or large-scale retrospective study regarding optimal treatment strategy and clinical outcomes of advanced gastric cancer perforation with invasion of adjacent organs. According to recent studies, the attempt to perform curative resection including lymphadenectomy whenever technically feasible might secure a good clinical outcome.578 However, the results were disappointing for advanced gastric cancer perforation, such as our case. One study group reported that emergent gastrectomy with secondary lymphadenectomy could increase the survival; however, they could not use this strategy in their 12 advanced gastric cancer cases.7 Another case series reported on 13 perforated gastric cancers, of which 5 were advanced gastric cancer; only one D2 lymph node dissection was possible5. The reported survival rate varies. Survival duration may be related to the stage of gastric cancer and the type of surgery performed.5789 A recent systematic review reported that one-year survival was 12.0% to 66.7% for noncurative gastrectomy, 26.6% to 80.3% for gastric resection, and 3.0% to 37.5% for surgical bypass or exploratory laparotomy.9 However, the included study groups were different with respect to cancer stage and preoperative condition; therefore, there seem to be no established data about the prognosis and clinical outcomes of perforated gastric cancer. Despite the lack of evidence, it seems reasonable to attempt curative resection to the extent possible to improve the clinical outcome. The concern is possible cancer cell dissemination; however, this might not significantly affect long-term survival in patients after gastrectomy.810
We contended with three problems in our case: 1) life-threatening sepsis; 2) wound management; 3) long-term survival. We drained the abscess preoperatively to prevent pus spillage into the peritoneal cavity. Surgery was for curative intent, although D2 lymph node dissection could not be performed due to hemodynamic instability. The patient received intensive treatment after surgery, including broad-spectrum antibiotics and total parenteral nutrition to treat pneumonia and sepsis, and to prevent surgery-related complications, such as leakage of the anastomosis. We prevented immediate postoperative complications and focused on wound management. Because there was no remaining tissue between the intra-abdominal organs and the skin in the left upper quadrant, we had no means of repairing the wound defect, including flap surgery, and the secondary healing of the wound was not anticipated. Instead, we protected the wound with wet dressings and applied an abdominal binder to prevent evisceration of the intra-abdominal organs, along with infection prevention. We planned to perform chemotherapy after improvement of her general condition; however, the growth of cancer progressed rapidly. On the 40th POD, the wound was filled with growing cancer and the patient died on the 82nd POD. The major concerns during treatment changed from life-saving and wound management to addressing goals of care.
There have been no established treatment guidelines for perforated gastric cancer with invasion to adjacent organs, and no reports on gastric cancer invading the abdominal wall. In such cases, we believe that drainage of pus before surgery can protect the peritoneal cavity from contamination, and might be helpful in performing curative resection.
Figures and Tables
Fig. 3
Operative findings reveal gastric cancer perforation and invasion through the entire abdominal wall.

References
1. Mahvi DM, Krantz SB. Stomach. In : Mahvi DM, Krantz SB, Townsend CM, Beauchamp RD, Evers BM, Mattox KL, editors. Sabiston textbook of surgery. Philadelphia: Elselvier Saunders;2012. p. 1217.
2. Bonenkamp JJ, Songun I, Hermans J, Sasako M, Welvaart K, Plukker JT, et al. Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet. 1995; 345:745–748.
3. Cuschieri A, Weeden S, Fielding J, Bancewicz J, Craven J, Joypaul V, et al. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Br J Cancer. 1999; 79:1522–1530.
4. Martin RC 2nd, Jaques DP, Brennan MF, Karpeh M. Extended local resection for advanced gastric cancer: increased survival versus increased morbidity. Ann Surg. 2002; 236:159–165.
5. Jwo SC, Chien RN, Chao TC, Chen HY, Lin CY. Clinicopathological features, surgical management, and disease outcome of perforated gastric cancer. J Surg Oncol. 2005; 91:219–225.
6. Heimlich HJ. The treatment of perforated cancer of the stomach. Am J Gastroenterol. 1963; 39:243–251.
7. Kasakura Y, Ajani JA, Fujii M, Mochizuki F, Takayama T. Management of perforated gastric carcinoma: a report of 16 cases and review of world literature. Am Surg. 2002; 68:434–440.
8. Kasakura Y, Ajani JA, Mochizuki F, Morishita Y, Fujii M, Takayama T. Outcomes after emergency surgery for gastric perforation or severe bleeding in patients with gastric cancer. J Surg Oncol. 2002; 80:181–185.
9. Mahar AL, Coburn NG, Singh S, Law C, Helyer LK. A systematic review of surgery for non-curative gastric cancer. Gastric Cancer. 2012; 15:Suppl 1. S125–S137.
10. Bonenkamp JJ, Songun I, Hermans J, van de Velde CJ. Prognostic value of positive cytology findings from abdominal washings in patients with gastric cancer. Br J Surg. 1996; 83:672–674.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download