Abstract
Hepatic metastasis of early gastric cancer (EGC) following subtotal gastrectomy with lymphadenectomy is rare. We report the case of a 61-year-old male patient who was diagnosed with EGC that was initially treated using endoscopic submucosal dissection (ESD) and subsequently underwent laparoscopic subtotal gastrectomy. Histopathological examination of the patient's ESD specimen showed a moderately differentiated tubular adenocarcinoma invading the submucosa without lymphatic invasion. The deep margin of the specimen was positive for adenocarcinoma, and he subsequently underwent laparoscopic distal gastrectomy. The patient developed liver metastasis 15 months after the operation and then underwent liver resection. Histology of the resected specimen confirmed the diagnosis of two foci of metastatic adenocarcinoma originating from stomach cancer. Immunohistochemical analysis of the specimen demonstrated overexpression of human epidermal growth factor receptor 2. The patient was treated with trastuzumab in combination with chemotherapy consisting of capecitabine and cisplatin. Twenty-four months after the operation, the patient remained free of recurrence.
Early gastric cancer (EGC) is defined as a lesion that is confined to the mucosa or submucosa regardless of the presence of lymph node metastasis. Endoscopic resection (ER) with endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) is a beneficial EGC treatment in that it is minimally invasive and preserves the stomach, allowing for improved quality of life.1
The recurrence rate of EGC following curative resection is reported to be between 1.3% and 13.8%.2 Hematogenous metastasis is the most common cause of relapse, and the liver is the most common site of recurrence with a reported incidence of 0.4% to 0.7%, followed by the lymph nodes and bones. Submucosal cancers are particularly prone to metastasis because of the abundance of vascular and lymphatic vessels in this space. Therefore, such lesions should be treated with gastrectomy and regional lymph node dissection regardless of margin involvement following ER.3
We report a case of metachronous liver metastasis in a patient with EGC initially treated by ESD. Since there was submucosal invasion of the primary lesion, the patient underwent a subsequent laparoscopic gastrectomy with regional lymph node dissection. There were no metastases to the regional lymph nodes at the time of resection. The lesion exhibited human epidermal growth factor receptor 2 (HER2) overexpression.
A 61-year-old man was admitted to our hospital for the evaluation and treatment of gastric cancer that was initially diagnosed by medical check-up. The patient's past medical history was unremarkable except for antihypertensive medication use. Laboratory findings were within their respective normal ranges, including levels of the tumor markers serum carcinoembryonic antigen, cancer antigen 19-9, and alpha fetoprotein.
Esophagogastroduodenoscopy revealed a 1.7-cm superficially elevated (IIa)-type of EGC in the gastric antrum on the lesser curvature (Fig. 1). This lesion was revealed as a moderately differentiated adenocarcinoma on pathological examination. Abdominopelvic computed tomography (CT) showed no evidence of distant metastasis. Given the clinical diagnosis of EGC, ESD was performed by a gastroenterologist. Histopathological examination of the specimen showed a moderately to poorly differentiated adenocarcinoma that invaded the submucosal layer to a depth of 1.5 mm (Fig. 2). The deep margin of the resected specimen had tumor involvement. There was no evidence of lymphatic, venous, or perineural invasion. We therefore performed a laparoscopic distal gastrectomy with lymph node dissection. There was no evidence of residual carcinoma in the resected specimen and no evidence of metastasis in the 38 identified lymph nodes. The postoperative course was uneventful, and the patient was discharged 14 days after the resection.
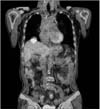
The patient was followed-up regularly, and a hepatic metastasis was diagnosed 15 months postoperatively. Abdominopelvic CT revealed a 3.5-cm low attenuation lesion in the dome of the liver and a second 1-cm lesion in segment 8 of the liver (Fig. 3). Positron emission tomography-computed tomography (PET-CT) failed to demonstrate any other metastatic disease, except in the liver (Fig. 4). Ultrasound-guided needle biopsy (TSK ACECUT biopsy needle; TSK Laboratory, Tochigi, Japan) of the liver lesions confirmed the diagnosis of a metastatic poorly differentiated adenocarcinoma. The patient underwent right anterior sectionectomy of the liver, and the specimen contained both lesions identified by imaging. Histopathological examination confirmed the diagnosis of two foci of metastatic adenocarcinoma consistent with a gastric primary (Fig. 5). Immunohistochemical analysis of the lesion demonstrated the presence of HER2. The patient was treated with trastuzumab in combination with chemotherapy consisting of capecitabine and cisplatin. Twenty-four months after the operation, the patient remained free of recurrence.
Lymph node metastasis is the most significant risk factor for EGC recurrence with a reported incidence of 1.4% to 2.8%.4 EGC patients with submucosal invasion have been reported to have a higher incidence of lymph node metastasis (23.8%). Unfortunately, preoperative diagnostic modalities for the detection of lymph node metastasis remain limited and inaccurate. Thus, when submucosal invasion is identified in patients who underwent ESD, it is prudent to consider potential curative resection since the possibility of residual tumor and/or lymph node metastasis after EMR/ESD cannot be ignored.1
Vascular invasion within the submucosal layer is a more significant risk factor for subsequent hepatic metastasis than lymph node or lymphatic invasion. Although venous invasion was not detected in this case, the venous invasion of primary tumor cells entering the portal circulation is presumed to be the cause of liver metastasis.3 EGC of the macroscopic elevated type is also associated with liver metastasis.3 Among EGCs, the dominant-elevated type has a relatively high incidence of vascular invasion thought to be due to tumor penetration and expansive growth.5
Hepatic resection for metastatic gastric cancer is not common due to the extremely poor postoperative prognosis. Therefore, liver resection would only benefit highly selected cases of hepatic metastasis from gastric cancer. Sakamoto et al.6 described criteria for considering resection of liver metastases in patients with gastric cancer. These include the absence of any distant metastatic disease including peritoneal dissemination and/or pulmonary involvement and the feasibility of macroscopic, complete gross resection.
In the present case, imaging (CT, magnetic resonance imaging, and PET-CT) revealed two lesions that were consistent with hepatic metastases. While only two metastatic lesions were identified, they were ultimately amenable to resection with a 5-mm safety margin. In patients with liver metastasis from colorectal cancer, liver resection with a tumor-free margin of less than 5 to 10 mm is sufficient because of the rare occurrence of satellite nodules around the main metastatic lesion.7 Meanwhile, in patients with hepatic metastases from gastric cancer the prognostic value of a particular surgical margin is controversial due to the aggressive biological behavior of the disease.
Overexpression of the HER2/neu proto-oncogene product is identified in 10% to 20% of gastric cancers and is correlated with poor outcome.8 An increased incidence of liver metastasis has been observed in patients with gastric cancer exhibiting HER2 overexpression. Dang et al.9 reported that 25.7% of gastric cancer patients with liver metastasis had tumors with HER2 overexpression. Trastuzumab, a recombinant anti-HER2 monoclonal antibody, acts synergistically with appropriate chemotherapy in the treatment of HER2-positive gastric cancer. In a recent international phase III clinical trial, the addition of trastuzumab to chemotherapy significantly improved overall patient survival compared with that of patients treated with chemotherapy alone.10 In this report, immunohistochemical analysis of ultrasound-guided needle biopsy specimens of the patient's metastatic liver mass demonstrated the presence of HER2. Therefore, our patient was treated with trastuzumab in combination with capecitabine and cisplatin. In a similar previously published case report, a small EGC removed by ESD showed submucosal invasion with lymphatic invasion and the patient subsequently underwent laparoscopic-assisted distal gastrectomy with lymph node dissection. One year later, liver resection was performed due to recurrence and the patient was treated with trastuzumab plus capecitabine/cisplatin because the resected specimen exhibited HER2 overexpression.11 In conclusion, liver metastasis rarely occurs in patients with EGC. Although there was no evidence of lymphatic, vascular, or perineural invasion in our case of EGC with submucosal invasion, the involvement of the deep margin and overexpression of HER2 potentially correlated with tumor recurrence.
Figures and Tables
Fig. 1
Esophagogastroduodenoscopy showing a small superficial elevated lesion in the antrum of the stomach.
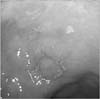
Fig. 2
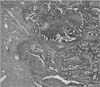
Histopathological examination of the specimens resected by endoscopic submucosal dissection revealing tubular adenocarcinoma with submucosal invasion (H&E, × 40).
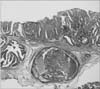
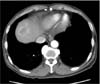
Fig. 3
An abdominopelvic computed tomography scan showing a 3.5-cm-mass in the liver that demonstrated strong enhancement following the intravenous administration of contrast material.
References
1. Hirasawa T, Gotoda T, Miyata S, Kato Y, Shimoda T, Taniguchi H, et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer. 2009; 12:148–152.
2. Youn HG, An JY, Choi MG, Noh JH, Sohn TS, Kim S. Recurrence after curative resection of early gastric cancer. Ann Surg Oncol. 2010; 17:448–454.
3. Ishida M, Morita S, Saka M, Fukagawa T, Taniguchi H, Katai H. Metachronous liver metastasis from early gastric cancer. J Gastrointest Surg. 2012; 16:837–841.
4. Saka M, Katai H, Fukagawa T, Nijjar R, Sano T. Recurrence in early gastric cancer with lymph node metastasis. Gastric Cancer. 2008; 11:214–218.
5. Kodama Y, Inokuchi K, Soejima K, Matsusaka T, Okamura T. Growth patterns and prognosis in early gastric carcinoma. Superficially spreading and penetrating growth types. Cancer. 1983; 51:320–326.
6. Sakamoto Y, Ohyama S, Yamamoto J, Yamada K, Seki M, Ohta K, et al. Surgical resection of liver metastases of gastric cancer: an analysis of a 17-year experience with 22 patients. Surgery. 2003; 133:507–511.
7. Yamamoto J, Sugihara K, Kosuge T, Takayama T, Shimada K, Yamasaki S, et al. Pathologic support for limited hepatectomy in the treatment of liver metastases from colorectal cancer. Ann Surg. 1995; 221:74–78.
8. Kim SY, Kim HP, Kim YJ, Oh do Y, Im SA, Lee D, et al. Trastuzumab inhibits the growth of human gastric cancer cell lines with HER2 amplification synergistically with cisplatin. Int J Oncol. 2008; 32:89–95.
9. Dang HZ, Yu Y, Jiao SC. Prognosis of HER2 over-expressing gastric cancer patients with liver metastasis. World J Gastroenterol. 2012; 18:2402–2407.
10. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastrooesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010; 376:687–697.
11. Namikawa T, Shiga M, Ichikawa K, Kitagawa H, Kobayashi M, Hanazaki K. Metachronous liver and bone metastasis from small early gastric carcinoma without lymph node involvement: a case report. Mol Clin Oncol. 2013; 1:249–252.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download