Abstract
Gastric cancer that mimics a submucosal tumor is rare. This rarity and the normal mucosa covering the protuberant tumor make it difficult to diagnosis with endoscopy. We report two cases of advanced gastric cancer that mimicked malignant gastrointestinal stromal tumors preoperatively. In both cases, the possibility of cancer was not completely ruled out. In the first case, a large tumor was suspected to be cancerous during surgery. Therefore, total gastrectomy with lymph node dissection was performed. In the second case, the first gross endoscopic finding was of a Borrmann type II advanced gastric cancer-like protruding mass with two ulcerous lesions invading the anterior wall of the body. Therefore, subtotal gastrectomy with lymph node dissection was performed. Consequently, delayed treatment of cancer was avoided in both cases. If differential diagnosis between malignant gastrointestinal stromal tumor and cancer is uncertain, a surgical approach should be carefully considered due to the possible risk of adenocarcinoma.
Gastric cancer that mimics a submucosal tumor (SMT) is rare, with a reported incidence of 0.1% to 0.63%.1 As the surface of the tumor is covered with mucosa that appears normal, it is difficult to obtain an adequate specimen from the underlying lesion. However, the differential diagnosis between a mesenchymal tumor, malignant lymphoma, and a carcinoid tumor is important to determine the optimal treatment strategy. In the treatment of locally advanced or borderline resectable gastrointestinal stromal tumors (GISTs), neoadjuvant imatinib has frequently resulted in regression or stabilization of the tumor. In addition, due to the rarity of lymphatic metastases in GIST, regional lymph node dissection is not performed. Herein, we report two cases of advanced gastric cancer (AGC) that mimicked malignant GIST and that were treated with palliative total gastrectomy and laparoscopy-assisted distal gastrectomy, respectively.
A 59-year-old woman was referred to Inje University Haeundae Paik Hospital for further examination of a 6 cm sized lobulated, hypoechoic mass, which was compressing the cardia and had been detected by ultrasonography at her local clinic in May 2010. Simple upper gastrointestinal endoscopic examination at the clinic revealed an extraluminal mass and chronic gastritis. At the initial outpatient examination, the patient's vital signs were stable with normal blood pressure and body temperature. The patient was a nonsmoker with an unremarkable medical history. Laboratory analysis revealed a hemoglobin level of 13.5 g/dl, a platelet count of 171,000/mm3, and partial thromboplastin time/activated partial thromboplastin time of 10.7/33.8 seconds. In addition, analysis showed an albumin level of 4.0 g/dl, a blood urea nitrogen level of 14.0 mg/dl, a creatinine level of 0.75 mg/dl, an aspartate transaminase level of 19 IU/L, an alanine transaminase level of 11 IU/L, a glucose level of 94 mg/dl, a carcinoembryonic antigen (CEA) level of 2.85 ng/ml, and the cancer antigen 19-9 level of 3.12 U/ml. There were no specific abnormal findings in the laboratory examination. However, a moderate-sized palpable mass in the epigastric area was noted on physical examination. Abdominal computed tomography (CT) revealed a lobulated soft tissue mass (7×6 cm) in the hepatogastric ligament and encasement of the left gastric artery abutting the gastric wall with thickening at the cardia and the upper body of the stomach (Fig. 1A). In addition, the mass abutted the pancreas, without evidence of direct invasion. The mass was suspected to be malignant GIST or exophytic gastric cancer. Therefore, upper gastrointestinal endoscopic examination was performed again in May 2010 to aid differential diagnosis. The examination revealed a protuberant tumor on the posterior wall of the upper body of the stomach, which was covered by mucosa that appeared normal except for slight ulceration (Fig. 1B). Endoscopic biopsy revealed chronic active gastritis with intestinal metaplasia. Clinically, the mass was diagnosed as a malignant SMT.
During the operation in May 2010, a tumor approximately 8 cm in size was observed on the lesser curvature of the upper body of the stomach, with invasion of the esophageal gastric junction and the body of the pancreas. There was no evidence of lymph node swelling, liver metastasis, peritoneal dissemination, or ascites. Palliative total gastrectomy with Roux-en-Y esophagojejunostomy and pancreas capsule resection was performed due to the high probability of malignancy. However, we were not convinced of microscopically complete resection even though we tried to perform a grossly complete resection, because a large hard mass had encased the celiac trunk and invaded the pancreas. According to gross examination, the tumor was Borrmann type IV. The serosal surface was grayish white and a protruding lesion measuring 7.0×5.5×2.5 cm was noted. The mucosa revealed a diffusely infiltrating lesion measuring 6.0×3.0 cm on the lesser curvature of the upper body of the stomach and the cut sections showed a grayish white mass infiltrating the perigastric fat tissue (Fig. 2). Histopathologically, the tumor had penetrated the serosa (T4b stage) and was a poorly differentiated tubular adenocarcinoma with regional metastasis in 3 out of 21 lymph nodes (N2 stage). The final diagnosis was of advanced gastric carcinoma with regional lymph node metastasis.
Positron emission tomography was performed to check for distant metastasis as soon as a pathological diagnosis of adenocarcinoma with lymph node metastasis was made on the 12th day after operation. It detected residual malignancy in the operation bed and axillary lymph node. No other postoperative complication was noted until the patient was discharged. However, endoscopic balloon dilatation was applied due to an anastomosis stricture a month after discharge. The patient underwent chemotherapy with tegafur plus gimeracil plus oteracil potassium (TS-1) immediately after recovering from surgery.
Follow-up CT scans were taken at intervals of about 3 months. However, multiple enlarged conglomerated lymph nodes around the celiac trunk were found on a follow-up CT in December 2011. Subsequently, the patient received second line chemotherapy (FOLFOX, 5-fluorouracil/leucovorin/oxaliplatin) and radiation therapy to the celiac trunk at a total dose of 4,500 cGy. Follow-up CT scans at shorter intervals of 3 months showed gradual improvement of the metastatic lymphadenopathy; the patient eventually had radiologically complete remission in October 2012. The patient has been in good condition except for mild dyspepsia up to the last follow-up in June 2014.
A 68-year-old man was referred to our hospital in January 2011 for differential diagnosis between AGC and malignant SMT. At a regular medical checkup, a Borrmann type II AGC-like protruding mass had been found at the gastric angle by simple upper gastrointestinal endoscopic examination in December 2010. However, endoscopic biopsy of the mass revealed only chronic gastritis. Initially, the patient's vital signs were stable with blood pressure and body temperature within normal limits. The patient was a nonsmoker and on medication for benign prostatic hyperplasia. However, he complained of persistent mild dyspepsia for a considerable period. Laboratory examination revealed a hemoglobin level of 12.15 g/ dl, a platelet count of 195,000/mm3, an albumin level of 4.1 g/ dl, a blood urea nitrogen level of 18.4 mg/dl, a creatinine level of 0.99 mg/dl, an aspartate transaminase level of 18 IU/L, an alanine transaminase level of 16 IU/L, a glucose level of 119 mg/dl, a CEA level of 8.54 ng/ml, cancer antigen 19-9 level of 24.07 U/ml, and a partial thromboplastin time/activated partial thromboplastin time of 10.7/33.8 seconds. There were no specific abnormal findings except the negligible anemia and increased CEA level. Normal active bowel sounds were heard and there was no palpable mass or tenderness on physical examination. A second upper gastrointestinal endoscopy examination was performed to confirm the lesion in January 2011. A large protruding ulcerative mass originating from the submucosa at the anterior wall of the lower body of the stomach and a small raised erosion at the cardia were observed (Fig. 3A). Histopathological diagnosis of a specimen from each lesion was of chronic active gastritis with intestinal metaplasia. Radiological findings via CT revealed a 4.8×1.7 cm sized submucosal gastric tumor with central ulceration involving the lesser curvature of the lower body of the stomach (Fig. 3B). There was no evidence of metastasis in any other organs. The clinical diagnosis was of a malignant SMT.
In March 2013, laparoscopic subtotal gastrectomy with lymph node dissection and Billroth II anastomosis was performed because a large tumor with two ulcerous lesions was found on the entire anterior wall of the body of the stomach. There was no visible evidence of lymph node swelling, liver metastasis, peritoneal dissemination, or ascites. However, the tumor was identified as Borrmann type I AGC on histopathological inspection. Multiple cut sections revealed a yellowish gray and myxoid mass infiltrating into the serosal layer and invading the muscularis propria (T2 stage). The tumor was mostly located within the submucosa and the muscle layer, with a very focal mucosal lesion. A deep infiltrating gland showed intraluminal mucin and an extracellular mucin pool in the subserosal layer (Fig. 4). The tumor was a mucinous carcinoma without metastasis to regional lymph nodes (N0 stage). The final diagnosis was of advanced gastric carcinoma without regional lymph node metastasis. The patient was discharged without any post-operative complications.
The patient underwent chemotherapy with TS-1 immediately after recovering from surgery. A CT scan taken 6 months after surgery showed no evidence of recurrence or metastasis. There has been no evidence of local recurrence in the follow-up CT scans and upper gastrointestinal endoscopy examinations at 6-month intervals up to the last follow-up in July 2014. The patient has been in good general condition after discharge.
Most gastric cancers are epithelial neoplasms that can be diagnosed by routine endoscopic biopsy. However, a gastric adenocarcinoma covered with normal-looking mucosa can macroscopically mimic a SMT on rare incidence (0.1%~0.63%).2 This rarity and the normal mucosa covering the protuberant tumor make it difficult to confirm diagnosis with endoscopy. Furthermore, this distinction is crucial in terms of therapeutic strategy. Treatment options for GIST include surgery and imatinib mesylate (Gleevec; Novartis, Basel, Switzerland), a competitive inhibitor of tyrosine kinases such as BCR-ABL, ARG, KIT, PDGFRa, and PDGFRb.3,4 Complete surgical resection is the most important factor with respect to survival in the treatment of GIST.5 However, routine lymph node dissection is unnecessary because lymphatic spread of GIST is very uncommon.6 In contrast, for all resectable T1b~T4 gastric cancers, optimal lymphadenectomy (D2 dissection) should be recommended as a standard of care.7
Large SMTs carry an increased risk of rupture, which has an unfavorable effect on disease-free and overall survival.8 Furthermore, according to a recent report, nearly all patients develop abdominal metastases following rupture of a GIST.9 However, imatinib induction therapy to reduce the size of large tumors could potentially reduce the risk of tumor rupture during surgery and provide an opportunity for a surgically complete and less morbid resection.10 Therefore, imatinib could be the first therapeutic option for large GISTs. However, imatinib is contraindicated for submucosal gastric cancer because it has no clinical effect on cancer and delays the diagnosis and treatment of cancer.
In the first case presented here, a large protuberant tumor was diagnosed as malignant SMT by radiological findings, and was diagnosed as chronic active gastritis with intestinal metaplasia twice by double-checked upper gastrointestinal endoscopic biopsy. However, prompt surgical intervention was performed because the possibility of gastric cancer had not been completely ruled out. We suspected the tumor to be gastric cancer during surgery. Therefore, total gastrectomy with lymph node dissection was performed.
In the second case, although the results of double-checked endoscopic biopsies indicated chronic active gastritis with intestinal metaplasia, the possibility of cancer was not completely ruled out, because the first gross endoscopic finding was a Borrmann type II AGC-like protruding mass. Furthermore, the large tumor with two ulcerous lesions invaded the entire anterior wall of the body of the stomach without serosal invasion. Additionally, the indication of laparoscopic gastrectomy for gastric cancer in our institute is T1N0, T1N1, and T2N0. Therefore, laparoscopic subtotal gastrectomy with lymph node dissection was performed.
In both cases, simple endoscopic biopsy was performed twice to aid the differential diagnosis. Unfortunately, the endoscopist in our institution did not perform a deep biopsy because of worries about perforation, bleeding, and delayed diagnosis. Therefore, the patient and their family were asked for informed consent prior to the operation because of the possibility of gastric cancer and SMT prior to operation.
Consequently, delayed treatment of cancer was avoided in both cases. If differential diagnosis between malignant GIST and cancer is uncertain, a surgical approach should be carefully considered due to the possible risk of adenocarcinoma.
Figures and Tables
Fig. 1
(A) Gastroscopic findings revealed a protuberant tumor on the posterior wall of the upper body and lesser curvature of the stomach. The tumor was covered by mucosa that appeared normal except for slight ulceration. (B) Computed tomography findings revealed a lobulated soft tissue mass in the hepatogastric ligament, and encasement of the left gastric artery abutting the gastric wall, with thickening in the cardia and the upper body of the stomach.

Fig. 2
(A) The serosal surface is grayish white and a protruding lesion measuring 7.0×5.5×2.5 cm was noted. The mucosa revealed a diffusely infiltrating lesion measuring 6.0×3.0 cm on the lesser curvature of the upper body of the stomach. (B) Staining method: H&E. Magnification: ×10 (left), ×200 (right).
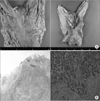
Fig. 3
(A) A large protruding ulcerative mass originating from the submucosa at the anterior wall of the lower body of the stomach and a single small raised erosion at the cardia. (B) Submucosal gastric mass at the lesser curvature of the gastric lower body (4.8 cm in segment and 1.7 cm in depth) with central ulceration.
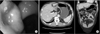
Fig. 4
(A) Multiple cut sections revealed a yellowish gray and myxoid mass measuring 5.8 × 3.0×1.4 cm, with infiltration of the serosal layer. The tumor was mostly located within the submucosa and the muscle layer, with a very focal mucosal lesion. A deep infiltrating gland showed intraluminal mucin and an extracellular mucin pool in the subserosal layer. (B) Staining method: H&E. Magnification: ×20 (left), ×100 (right).

Acknowledgments
This abstract was accepted as a poster presentation during The 32nd Annual Congress of The Korean Gastric Cancer Association in Busan (abstract ID: PIII-18).
References
1. Umehara Y, Kimura T, Okubo T, Sano Y, Nakai K, Oi S, et al. Gastric carcinoma resembling submucosal tumor. Gastric Cancer. 1999; 2:191–193.
2. Burkill GJ, Badran M, Al-Muderis O, Meirion Thomas J, Judson IR, Fisher C, et al. Malignant gastrointestinal stromal tumor: distribution, imaging features, and pattern of metastatic spread. Radiology. 2003; 226:527–532.
3. Hasegawa T, Matsuno Y, Shimoda T, Hirohashi S. Gastrointestinal stromal tumor: consistent CD117 immunostaining for diagnosis, and prognostic classification based on tumor size and MIB-1 grade. Hum Pathol. 2002; 33:669–676.
4. Heinrich MC, Griffith DJ, Druker BJ, Wait CL, Ott KA, Zigler AJ. Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood. 2000; 96:925–932.
5. Schwameis K, Fochtmann A, Schwameis M, Asari R, Schur S, Köstler W, et al. Surgical treatment of GIST: an institutional experience of a high-volume center. Int J Surg. 2013; 11:801–806.
6. Morinaga N, Sano A, Katayama K, Suzuki K, Kamisaka K, Asao T, et al. Laparoscopic transgastric tumor-everting resection of the gastric submucosal tumor located near the esophagogastric junction. Surg Laparosc Endosc Percutan Tech. 2004; 14:344–348.
7. Orditura M, Galizia G, Sforza V, Gambardella V, Fabozzi A, Laterza MM, et al. Treatment of gastric cancer. World J Gastroenterol. 2014; 20:1635–1649.
8. Ng EH, Pollock RE, Munsell MF, Atkinson EN, Romsdahl MM. Prognostic factors influencing survival in gastrointestinal leiomyosarcomas. Implications for surgical management and staging. Ann Surg. 1992; 215:68–77.
9. Hohenberger P, Ronellenfitsch U, Oladeji O, Pink D, Ströbel P, Wardelmann E, et al. Pattern of recurrence in patients with ruptured primary gastrointestinal stromal tumour. Br J Surg. 2010; 97:1854–1859.
10. Eisenberg BL, Harris J, Blanke CD, Demetri GD, Heinrich MC, Watson JC, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/ recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol. 2009; 99:42–47.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download