Abstract
Purpose
The role of peritoneal washing cytology in determining further treatment strategies after surgery for gastric cancer remains unclear. One reason for this is the fact that optimal procedures to increase the accuracy of predicting peritoneal metastasis have not been established. The aim of this study was to evaluate the efficacy of cytology using samples harvested from two different abdominal cavity sites during gastric cancer surgery.
Materials and Methods
We prospectively recruited 108 patients who were clinically diagnosed with locally advanced gastric cancer (higher than cT1 stage disease). Peritoneal washing fluids were collected from the pouch of Douglas and the subphrenic area. Patients were prospectively followed up for 2 years to determine the recurrence and survival rates.
Results
Thirty-three patients dropped out of the study for various reasons, so 75 patients were included in the final analysis. Seven patients (9.3%) showed positive cytology findings, of whom, three showed peritoneal recurrence. Tumor size was the only factor associated with positive cytology findings (P=0.037). The accuracy and specificity of cytology for predicting peritoneal recurrence were 90.1% and 94.2%, respectively, whereas the sensitivity was 50.0%. The survival rate did not differ between patients with positive cytology findings and those with negative cytology findings (P=0.081).
Conclusions
Peritoneal washing cytology using samples harvested from two different sites in the abdominal cavity was not able to predict peritoneal recurrence or survival in gastric cancer patients. Further studies will be required to determine whether peritoneal washing cytology during gastric cancer surgery is a meaningful procedure.
Although the incidence of gastric cancer has declined recently, gastric cancer remains one of the most common causes of death due to malignant tumors worldwide.1 A major cause of gastric cancer-associated mortality in patients who undergo curative resection is recurrent disease, with the most common site of recurrence being the peritoneum.2 To date, multimodal treatment strategies have been used to improve the prognosis of gastric cancer patients with peritoneal recurrence, but the results remain unsatisfactory.3 Therefore, it is important to prevent peritoneal recurrence after curative surgery to improve the prognosis of gastric cancer patients. The recent trend in treatment is the administration of adjuvant intraperitoneal chemotherapy immediately after resection in patients who are at high risk for peritoneal recurrence.4,5 However, to apply this modality, selection of patients who are at high risk for peritoneal recurrence is crucial. Although the precise mechanism driving peritoneal recurrence remains unclear, the presence of malignant cells in the peritoneum at the time of surgery can lead to peritoneal recurrence.6,7 Therefore, examination of peritoneal fluids has emerged as an option for identifying patients who are at high risk for peritoneal recurrence after curative resection.
Several previous studies have reported that examination of the peritoneum for free malignant cells is effective for predicting peritoneal metastasis from gastric cancer.8,9 Moreover, this approach does not require any additional procedures and is non-invasive since it can be performed concurrently with the resection operation. On the basis of these clinical results and because of the ease of performance of the procedure, positive cytology findings are considered to represent distant metastasis according to the gastric cancer classification system proposed by the Japanese Gastric Cancer Association (JGCA).10 Furthermore, the recent guidelines of the TNM staging system of the American Joint Committee for Cancer (AJCC) also consider positive peritoneal cytology as a sign of metastatic disease.11 However, cytology is not yet routinely performed during gastric cancer surgery because the reported accuracy and sensitivity of cytology vary, being low in some studies.12 Therefore, optimal procedures to increase the efficacy and reproducibility of this modality need to be established. To address this issue, we conducted a prospective study with the aim of increasing the efficacy and reproducibility of cytology using washing samples collected from multiple peritoneal sites such as the pouch of Douglas and the upper abdomen.
The aims of this prospective study were to determine the prevalence of positive cytology findings using samples from two peritoneal sites in gastric cancer patients and to investigate the efficacy of cytology in predicting peritoneal recurrence of gastric cancer. On the basis of these results, we sought to determine the necessity of cytology during gastric cancer surgery.
This prospective study was approved by the institutional review board at Ajou University Hospital, Suwon, Korea (IRB No: MED-SMP-10-096) and conformed to the ethical guidelines outlined in the Declaration of Helsinki (1975). Patients were enrolled into a single study arm from January 2010 through December 2010 at Ajou University Hospital. Written informed consent was obtained from each patient prior to enrollment. The primary endpoint was the rate of positive results for malignancy using the peritoneal washing samples of patients who were clinically and surgically diagnosed with resectable advanced gastric cancer. We also investigated the clinicopathological characteristics and 3-year survival rate associated with positive cytology findings as secondary endpoints.
We enrolled patients who fulfilled the following inclusion criteria: advanced gastric adenocarcinoma (invasion of the muscularis propria or beyond by primary tumors) without distant metastasis as determined by preoperative physical examination and imaging studies and a normal physiologic condition, defined as an American Society of Anesthesiology physical status score of 3 or lower. Patients with concurrent malignancies, those with a possibility of pregnancy, and those participating in other clinical trials were excluded from the study.
After enrollment in the current study, some patients were determined during surgery to have early-stage gastric cancer or previously undetected metastatic lesions and were consequently withdrawn from the study. In addition, some patients withdrew from the study for their own reasons and were therefore excluded from the final analyses.
The surgical parameters, such as the methods for gastric resection and the extent of lymph node dissection, were determined according to JGCA treatment guidelines (3rd edition).13 At our institution, laparoscopic surgery is usually performed for patients observed to have a primary tumor invading up to the muscularis propria during preoperative examination.
Peritoneal washing samples were cytologically examined prior to tumor mobilization, immediately after laparotomy or laparoscopic examination, with patients under general anesthesia. Saline (200 ml) was poured into the pouch of Douglas and stirred. Then, 50 to 100 ml of the fluid was collected for cytological examination using an aspiration tube. Another 200 ml of saline was subsequently introduced into the upper abdomen, and fluid was collected from the left subphrenic area. Collected samples were immediately sent to the Department of Pathology, after which, the surgeon continued with the gastric cancer surgery. The fluids were centrifuged, and the cell pellets were examined microscopically by an experienced pathologist using Papanicolaou and Giemsa staining.
Upon cytological examination, sample findings were classified by the pathologist as negative, atypical (indeterminate), suspicious, or definitive for malignancy according to conventional cytology criteria for interpretation.14 The diagnosis of definite malignancy was based on findings of abnormal mitotic figures as well as cells with large, hyperchromatic, irregularly shaped nuclei and irregular cytoplasmic vacuoles on Papanicolaou and Giemsa staining. When specimens showed some features of definite malignancy, findings were classified as suspicious. Atypical findings referred to the presence of any kind of abnormal cells other than definite or suspicious malignant cells in the fluid. For the purpose of this study, when one or both fluid samples from the two peritoneal sites had suspicious findings or findings that were definitive for malignancy, the sample was considered as being positive for malignancy (Fig. 1); all other findings were considered as negative for malignancy. The resected specimens were also examined by the pathologist and were classified according to the AJCC TNM classification (7th edition)11 and the Japanese Classification of Gastric Carcinoma.10
The patients were followed up for a mean duration of 30 months after surgery, during which time, they were regularly evaluated every 3 to 6 months. The evaluations included patient history taking, physical examination, computed tomography (CT) of the abdominopelvic area, and measurement of tumor markers. Patients with a histological diagnosis of advanced gastric cancer were administered 5-fluorouracil-based chemotherapy as adjuvant treatment. The types of regimens, doses, and durations varied according to the patients' conditions.
During the follow-up period, CT was performed to detect recurrence in non-symptomatic patients. Patients in whom recurrence was suspected on CT underwent additional magnetic resonance imaging to detect liver metastasis and laparoscopic exploration to detect peritoneal seeding. Recurrence was classified as distant lymph node recurrence, hematogenous spread, or peritoneal metastasis, according to the metastatic route(s). When the recurrent mass caused bowel obstruction, an endoscopic stent was inserted or bypass surgery was performed. Among patients with recurrence, those with a favorable condition received systemic chemotherapy with a palliative intent.
The sample size was calculated using G-Power version 3.1.9 for Mac OS (Heinrich Heine University, Dusseldorf, Germany). The proportion of samples with positive cytology findings on using the conventional method (sampling only from the pouch of Douglas) was approximately 10% in a previous report.9 The proportion of samples with positive cytology findings on using our detection methods (sampling from two different sites) was assumed to be 20%. The type I error was set as 0.1 (one sided) and the power was 90%. The sample size was calculated accordingly considering the use of the chi-square test. A sample size of 108 was required to detect a significant increase in the positive cytology rate in our study compared to the previous report, accounting for a 25% dropout rate.
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS), version 21 for Mac OS (IBM Corp., Armonk, NY, USA). Categorical data were analyzed using the chi-square test or Fisher's exact test. Survival was assessed using the Kaplan-Meier method and was compared between groups using the log-rank test. A value of P<0.05 was considered statistically significant.
A total of 108 patients were initially enrolled in the current study. However, 12 patients (11.1%) were subsequently found to have previously undetected metastatic lesions or a non-resectable extension of the primary tumor during surgery. In addition, 15 patients (13.9%) with resectable lesions were found to have early-stage gastric cancer during surgery, and six other patients were lost to follow-up in 1 year or less after surgery, without confirmation of the recurrence status or death. Another 33 patients withdrew from the study, resulting in a total of 75 patients for whom peritoneal washing cytology was finally performed followed by gastric resection with lymph node dissection (Fig. 2).
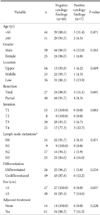
Background characteristics of the patients are listed in Table 1. Positive cytology results were those associated with suspicious or definitive findings for malignancy in washing fluids collected from the pouch of Douglas or the subphrenic area. The only clinicopathological finding that was observed to be associated with positive cytology findings was a tumor size of ≥5 cm (P=0.037). During the follow-up period (mean, 30 months), 18 patients (24%) showed recurrence. The most common sites of recurrence were the peritoneum (n=6) and the distant lymph nodes (n=6), with other sites including the locoregional area, the liver, and the lungs. The disease-free survival rate did not differ according to cytology results (P=0.210), nor did the overall survival rate (P=0.081; Fig. 3).
Of the 75 patients, seven (9.3%) had positive cytology findings (suspicious or definitive for malignancy). Two of the seven patients had definitive results for malignancy upon cytological examination of washing fluids from both the pouch of Douglas and the subphrenic area. One of these two patients was diagnosed with peritoneal recurrence 6.7 months after surgery, but the other survived without recurrence during the two years of follow-up. Two other patients with positive cytology results had definitive results for malignancy based only on the fluid from the pouch of Douglas, whereas the fluid harvested from the subphrenic area of these patients showed suspicious results. These two patients demonstrated confirmed peritoneal recurrence 7.2 and 18.4 months after surgery, respectively. Thus, three of the four patients with definitive results for malignancy experienced peritoneal recurrence. The other three patients with recurrence had suspicious results for malignancy based on fluids collected from both sites. Although these patients have been followed up over 2 years, none have been diagnosed with recurrence in the peritoneum or at any other site. Additional clinicopathological features of patients with positive cytology results are listed in Table 2.
We sought to determine the differences between definitive and suspicious cytology results for malignancy in terms of the accuracy of predicting peritoneal recurrence. When positive results were defined as suspicious or definitive results for malignancy, the accuracy was 90.1%. When the criterion was limited to definitive results for malignancy, the accuracy increased slightly to 93.3% (Table 3). The sensitivity, specificity, and predictive values of cytology, for assessing its diagnostic value, are listed in Table 3. In contrast with other metrics, the positive predictive value significantly increased from 42.9% to 75.0% when the criterion for positive cytology findings was limited to definitive results for malignancy.
In the present prospective study, we found that cytology was associated with a low sensitivity and positive predictive value for predicting peritoneal recurrence in patients with gastric cancer, despite the fact that we performed cytology using samples collected from two different abdominal cavity sites and expanded the definition of positive cytology findings to include suspicious results. One patient diagnosed with positive cytology finding did not develop peritoneal recurrence. In addition, the survival rate did not differ between patients with positive and negative cytology findings. Our results suggest that routine cytology during curative surgery for gastric cancer is an unnecessary procedure.
Different studies have reported different rates of positive peritoneal cytology findings in gastric cancer. In a Dutch randomized prospective clinical trial comparing D1 and D2, positive cytology findings were detected in only 12% of patients who were pathologically diagnosed with serosa-exposed primary tumors (stage pT4 or higher).15 Meanwhile, other studies have reported rates of positive cytology findings ranging from 15.7% to 35.0%.8,16,17,18 These variable rates can be attributed to the pathologic differences among the recruited patients. Patients with primary tumors not involving the serosa had a positive cytology finding rate of 1.0% or less, whereas this rate was 20% or higher among patients with tumors involving the serosa.8,9 In our study, the rate of positive cytology findings was relatively low (9.3%) compared to previous reports, despite the fact that we collected samples from multiple sites. Our study was designed as a prospective study, and we therefore attempted to enroll patients determined to have locally advanced gastric cancer on preoperative evaluations such as CT and gastroscopy and excluded those with morphologic characteristics of early gastric cancer in the surgical field. Nevertheless, 15 (20%) of the enrolled 75 patients had early-stage primary tumors (T1), which may have contributed to the low rate of positive cytology findings in our study, compared to previous studies. When we subclassified patients according to pathologic tumor invasion, the rate of positive cytology findings in patients with primary tumors involving the serosa was 22.7%, comparable to the results of previous studies.
According to most previous reports, the sensitivity of cytology for predicting peritoneal recurrence is relatively lower (ranging from 11% to 43%) than the corresponding specificity.8,9,19,20 This low sensitivity indicates that many patients with negative findings on peritoneal cytology can develop peritoneal recurrence. We collected samples from two different sites to increase the sensitivity of cytology in our study, but the results were disappointing. Of the patients who developed peritoneal recurrence, 50% had negative peritoneal cytology findings. To date, several efforts have been made to increase the sensitivity of cytology for predicting peritoneal recurrence in gastric cancer. Hayes et al.21 reported that collection of samples for cytology using peritoneal brushing could increase the detection rate of free malignant cells in the peritoneum. In another study, cytology was performed by directly rinsing the surface of the primary tumor with saline.22 However, this might provoke undesirable exfoliation of tumor cells from the primary tumor involving the serosal surface, which could potentially decrease the specificity due to false-positive results. In our study, we also did not observe a satisfactory sensitivity, despite checking samples from two common sites of peritoneal metastasis: the subphrenic area and the pouch of Douglas.
Another method for improving the sensitivity of cytology in gastric cancer is to check for the presence of molecular markers using immunohistochemistry or reverse transcription polymerase chain reaction (RT-PCR). Immunohistochemical staining with, for example, Ber-EP4 or HEA-125, has been performed to detect free cancer cells in the peritoneum, but this method has also been found to have a low sensitivity, as is the case with conventional cytology.20,23 Alternatively, when RT-PCR was used to detect molecular markers such as carcinoembryonic antigen (CEA), cytokeratin 20, and matrix metallopeptidase 7 in peritoneal fluid, the sensitivity significantly increased, compared to other methods.24,25,26,27 Tokuda et al.27 reported that RT-PCR for CEA had a sensitivity of 93.8% and a specificity of 87.5% for predicting peritoneal recurrence. However, the detection of molecular markers could result in an increased false-positive rate. Moreover, this is a very time-consuming procedure with no evidence to indicate its cost-effectiveness. Nevertheless, if additional clinical evidence indicating that the detection of molecular markers can be used to predict peritoneal recurrence emerges, this option may be further explored in the future.
In the current study, patients with positive cytology findings did not have a significantly poorer prognosis than those with negative cytology findings. Most previous studies have reported that positive cytology findings could be a predictive factor for poor prognosis.8,20,23,24,25 This may not have been the case in our study for several reasons. First, the patients with positive cytology findings in our study received aggressive systemic chemotherapy (as listed in Table 2). Those with advanced gastric cancer were postoperatively treated with 5-fluorouracil-based chemotherapy, pursuant to the results of recent clinical studies.28,29,30 These treatments might mitigate any differences in prognosis according to the cytology results. In addition, 66% of patients with negative cytology had primary tumors invading the subserosa or serosal layer. Because of the advanced stage of disease in a large proportion of patients with negative cytology findings, these patients did not display a better prognosis than patients with positive cytology findings. In addition, three patients who did not show recurrence despite positive cytology findings have been observed for a period of approximately 24 months following surgery in this study. Although the mean time to peritoneal recurrence after surgery has been reported to be 16 to 18 months and more than 80% of peritoneal recurrences have been reported to be detected within 2 years after surgery,2,31 we will need to follow up these patients over 5 years to confirm the value of positive cytology findings as a prognostic factor.
Taken together, our findings suggest that peritoneal washing cytology had a low sensitivity for predicting peritoneal recurrence in patients with clinically and surgically determined advanced gastric cancer. Despite using samples collected from two different abdominal cavity sites, the observed rate of positive peritoneal cytology finding was not higher than expected. Moreover, it was not clear from our study whether peritoneal cytology findings are appropriate for predicting prognosis in patients. However, our study did not show the results of long-term follow-up of the recruited patients, and the study sample size was calculated according to the rate of positive findings for malignancy on cytology, instead of according to the significance of survival differences or the differential accuracy of predicting peritoneal recurrence according to cytology results. Therefore, we could not determine whether peritoneal cytology should be completely excluded in gastric cancer surgery. Further studies with greater numbers of patients or additional molecular analysis could make this procedure more meaningful in the future.
Figures and Tables
Fig. 1
Isolated carcinoma cells showing hyperchromatic crescent-shaped nuclei and vacuolated cytoplasm (Papanicolaou stain, ×400).

Fig. 3
Survival rates of patients enrolled in the study. Overall survival rate (A) and disease-free survival rate (B) according to cytology results. The P-value was calculated using the log-rank test.

Acknowledgments
This research was supported by the New Faculty Research Fund of Ajou University School of Medicine.
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.

2. Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000; 87:236–242.

3. Glockzin G, Piso P. Current status and future directions in gastric cancer with peritoneal dissemination. Surg Oncol Clin N Am. 2012; 21:625–633.

4. Matharu G, Tucker O, Alderson D. Systematic review of intraperitoneal chemotherapy for gastric cancer. Br J Surg. 2011; 98:1225–1235.

5. Yan TD, Black D, Sugarbaker PH, Zhu J, Yonemura Y, Petrou G, et al. A systematic review and meta-analysis of the randomized controlled trials on adjuvant intraperitoneal chemotherapy for resectable gastric cancer. Ann Surg Oncol. 2007; 14:2702–2713.

6. Kojima N, Kunieda K, Matsui K, Kato H, Saji S. Evaluation of carcinoembryonic antigen mRNA in living, necrotic, and apoptotic gastric cancer cells by reverse transcriptase-polymerase chain reaction. Surg Today. 2003; 33:839–846.

7. To EM, Chan WY, Chow C, Ng EK, Chung SC. Gastric cancer cell detection in peritoneal washing: cytology versus RT-PCR for CEA transcripts. Diagn Mol Pathol. 2003; 12:88–95.

8. Euanorasetr C, Lertsithichai P. Prognostic significance of peritoneal washing cytology in Thai patients with gastric adenocarcinoma undergoing curative D2 gastrectomy. Gastric Cancer. 2007; 10:18–23.

9. Kodera Y, Yamamura Y, Shimizu Y, Torii A, Hirai T, Yasui K, et al. Peritoneal washing cytology: prognostic value of positive findings in patients with gastric carcinoma undergoing a potentially curative resection. J Surg Oncol. 1999; 72:60–64. discussion 64-65.

10. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011; 14:101–112.
11. Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM classification of malignant tumors. 7th ed. Hoboken: Wiley-Blackwell;2009.
12. Leake PA, Cardoso R, Seevaratnam R, Lourenco L, Helyer L, Mahar A, et al. A systematic review of the accuracy and utility of peritoneal cytology in patients with gastric cancer. Gastric Cancer. 2012; 15:Suppl 1. S27–S37.

13. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011; 14:113–123.
14. Bernard N. Pleural, peritoneal and pericardial effusion. In : Bibbo M, editor. Comprehensive Cytopatholgy. 3rd ed. Philadelphia: Saunders;2008. p. 549–552.
15. Bonenkamp JJ, Songun I, Hermans J, van de Velde CJ. Prognostic value of positive cytology findings from abdominal washings in patients with gastric cancer. Br J Surg. 1996; 83:672–674.

16. Abe S, Yoshimura H, Tabara H, Tachibana M, Monden N, Nakamura T, et al. Curative resection of gastric cancer: limitation of peritoneal lavage cytology in predicting the outcome. J Surg Oncol. 1995; 59:226–229.

17. Boku T, Nakane Y, Minoura T, Takada H, Yamamura M, Hioki K, et al. Prognostic significance of serosal invasion and free intraperitoneal cancer cells in gastric cancer. Br J Surg. 1990; 77:436–439.

18. Ikeguchi M, Oka A, Tsujitani S, Maeta M, Kaibara N. Relationship between area of serosal invasion and intraperitoneal free cancer cells in patients with gastric cancer. Anticancer Res. 1994; 14:2131–2134.
19. Sugita Y, Fujiwara Y, Taniguchi H, Mori T, Ishii T, Niwa H, et al. Quantitative molecular diagnosis of peritoneal lavage fluid for prediction of peritoneal recurrence in gastric cancer. Int J Oncol. 2003; 23:1419–1423.

20. Vogel P, Rüschoff J, Kümmel S, Zirngibl H, Hofstädter F, Hohenberger W, et al. Immunocytology improves prognostic impact of peritoneal tumour cell detection compared to conventional cytology in gastric cancer. Eur J Surg Oncol. 1999; 25:515–519.

21. Hayes N, Wayman J, Wadehra V, Scott DJ, Raimes SA, Griffin SM. Peritoneal cytology in the surgical evaluation of gastric carcinoma. Br J Cancer. 1999; 79:520–524.

22. Wu CC, Chen JT, Chang MC, Ho WL, Chen CY, Yeh DC, et al. Optimal surgical strategy for potentially curable serosa-involved gastric carcinoma with intraperitoneal free cancer cells. J Am Coll Surg. 1997; 184:611–617.
23. de Manzoni G, Verlato G, Di Leo A, Tomezzoli A, Pedrazzani C, Pasini F, et al. Peritoneal cytology does not increase the prognostic information provided by TNM in gastric cancer. World J Surg. 2006; 30:579–584.

24. Fujii S, Kitayama J, Kaisaki S, Sasaki S, Seto Y, Tominaga O, et al. Carcinoembryonic antigen mRNA in abdominal cavity as a useful predictor of peritoneal recurrence of gastric cancer with serosal exposure. J Exp Clin Cancer Res. 2002; 21:547–553.
25. Hara M, Nakanishi H, Jun Q, Kanemitsu Y, Ito S, Mochizuki Y, et al. Comparative analysis of intraperitoneal minimal free cancer cells between colorectal and gastric cancer patients using quantitative RT-PCR: possible reason for rare peritoneal recurrence in colorectal cancer. Clin Exp Metastasis. 2007; 24:179–189.

26. Yonemura Y, Fujimura T, Ninomiya I, Kim BS, Bandou E, Sawa T, et al. Prediction of peritoneal micrometastasis by peritoneal lavaged cytology and reverse transcriptase-polymerase chain reaction for matrix metalloproteinase-7 mRNA. Clin Cancer Res. 2001; 7:1647–1653.
27. Tokuda K, Natsugoe S, Nakajo A, Miyazono F, Ishigami S, Hokita S, et al. Clinical significance of CEA-mRNA expression in peritoneal lavage fluid from patients with gastric cancer. Int J Mol Med. 2003; 11:79–84.

28. Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. CLASSIC trial investigators. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012; 379:315–321.

29. Nakajima T, Kinoshita T, Nashimoto A, Sairenji M, Yamaguchi T, Sakamoto J, et al. National Surgical Adjuvant Study of Gastric Cancer Group. Randomized controlled trial of adjuvant uracil-tegafur versus surgery alone for serosa-negative, locally advanced gastric cancer. Br J Surg. 2007; 94:1468–1476.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download