Abstract
Purpose
Laparoscopic wedge resection of gastric submucosal tumor may be difficult in case of the endophytic mass or the mass located unreachable area such as cardia, and intragastric approach can be useful. We would present the experiences of the intragastric wedge resection.
Materials and Methods
There were 7 patients diagnosed as gastric submucosal tumor and underwent the intragastric wedge resection at Surgery, Chungnam National University Hospital. We reviewed medical record.
Results
There were 3 male and 4 female. Mean age was 65 years-old (57~73). Mean body mass index was 26.28 kg/m2 (21.28~35.30). Location of lesions was 4 cardia, 2 fundus and 1 midbody, respectively. Mean operation time was 83.6 minutes (70~105). All patients were healed without any complication. Mean postoperative hospital stay was 5.4 days (4~6). Mean size was 2.7 cm (2.3~3.8). Pathologic finding was 5 gastrointestinal stromal tumor and 2 leiomyoma.
The surgical resection of the gastric submucosal tumor (SMT) is needed because the pathology can be malignant and the preoperative diagnosis is difficult with any modality including the endoscopic ultrasound-fine needle aspiration biopsy, multidirectional computed tomography and immunocytochemical and molecular analyses.(1,2) About 80% of SMT is benign, Gastrointestinal stromal tumor (GIST) account for 1% of all gastrointestinal neoplasms and are the most common mesenchymal tumor of gastrointestinal tract. GIST has benign behavior in small size but also is getting malignancy along the increasing size. The national comprehensive cancer network and European society of medical oncology recommended the tumor ≥2 cm should be resected.(3) But the incidence of the SMT was increased with advance of diagnostic tools, and in the Canadian guidelines, the tumor <1 cm should be resected because the small size cannot guarantee a specific malignant risk for gastric SMT.(4,5) So there is a need to remove even the small SMT.
The minimally invasive approaches have been increasingly needed as technical advances of laparoscopic surgery have been established. The laparoscopic wedge resection for small and medium sized GISTs is known as the oncologically safe and technically feasible method.(6-10) It is prior to the open surgery even in the cosmesis and short-term postoperative outcomes such as pain. Despite of these advantages, the laparoscopic wedge resection has the limitation such as location. In case of high lying gastric SMT, especially located in the posterior wall or near the esophagogastric junction, the operation is complicated because the dissection of the stomach along the greater curvature or the formation of a gastrostomy at the anterior wall is necessary. The intragastric wedge resection may be beneficial in this case, especially when the tumor is located in the fundus, cardia or close to the esophagogastric junction (EGJ).(11-14) The advantages of a single incision intragastric approach are considered (1) direct visualization of the tumor during the resection compared to conventional laparoscopic wedge resections for endophytic tumors, (2) ease of tumor delivery through a single incision site, and (3) extracorporeal repair of gastrostomy sites. To verify of these advantages, we tried a single incision intragastric approach and report operative outcomes for 7 cases.
Single incision laparoscopic intragastric wedge resection for gastric submucosal tumor was performed in 7 patients who gave written informed consent between June 2009 and April 2011 at Chungnam National University Hospital. Preoperative diagnosis was made by endoscopic ultrasonography with endoscopic punch biopsy. Once we obtained the initial endoscopic finding for the SMT, all patients were examined by computerized tomography (CT) to determine the depth of invasion, the possibility of a malignant GIST, and distant metastasis. Hospital records of all patients were examined retrospectively regarding basic demographics, operation time, hospital stay, time to resuming first sips of water, immediate postoperative complications, and pathologic results.
The patient was placed in the supine position under the general anesthesia. The surgeon stood on the right side of the patient. A first assistant stood on the left side and made an umbilical incision. After a midline umbilical incision was made (3 cm in length), an extra small Alexis wound retractor (Applied Medical, Rancho Santa Margarita, CA, USA) was applied to the incision. The stomach was then brought out though the incision and opened with an electrosurgical device that was 2 cm in length. A 4 channel OCTO™ port V2-B (Dalim, Seoul, Korea) was applied to the gastric opening (Fig. 1). After inflation of the stomach with CO2, exploration of the gastric mucosa was done. The pressure of CO2 for inflation was about 10 mmHg. The laparoscope was 30 degree, 10 mm diameter. Once the tumors were detected, they were pulled with a curved grasper (Cambridge Endo, Framingham, MA, USA). To prevent the rupture and dissemination of the tumor cell into the peritoneal cavity, first the normal mucosal adjacent to the tumor was grasped and resected with a 45 mm Endo-GIA (Tyco Healthcare, Covidien, Elancourt, France). Then the resected mucosa was pulled up continually, the remained tumor was resected with a 45 mm Endo-GIA little by little. After specimen is put in the lap-bag, the specimen was obtained through the gastric incision and the umbilical incision. After the OCTO™ port was removed, the gastric opening was closed by a continuous interlocking full layer suture and seromuscular reinforcement was done with absorbable suture material. A drain was placed on a case by case basis. The patients were always given the proton-pump inhibitor intravenously or orally after surgery.
Seven SMTs diagnosed by endoscopic ultrasound, were resected by single incision laparoscopic intragastric wedge resection. All of the resections were performed by a single, experienced surgeon using laparoscopic surgery. There were 4 male patients and 3 female patients. Mean age of the patients was 65 years (range, 57~73) (Table 1). The mean body mass index was 26.28 kg/m2 (range, 21.28~35.30). One patient was diagnosed with diabetes mellitus and 5 with hypertension. No patient had a history of a major operation and the American society of anesthesiologist scores were all below 3. The mean diameter of tumors based on pathologic reports was 2.7 cm (range, 2.3~3.8 cm). Most tumors were located in the proximal stomach (cardia 4, fundus 2, lower body 1). In the pathologic report, 5 SMTs were diagnosed as GISTs and 2 as leiomyomas. There were two malignant GISTs (Table 2). The median period of follow up was 8.5 months and there was no disease recurrence on endoscopy and on CT. One patient had complained minor symptoms such as dyspepsia and postprandial pain.
Mean operating time was 83.6 minutes (70 to 105). There was no intraoperative complication and no conversion to open surgery. The day of resuming first liquid diet was at 2.8 days postoperatively (range, 2~3). The average postoperative hospital stay was 5.7 days (range, 4~7). Until the third postoperative day, abdominal pain was controlled by intravenous non-steroidal anti-inflammatory drugs; 3 patients needed additional analgesics. Postoperative complications included one case of wound bleeding. The number of consumed Endo-GIA to resect the tumor was 3 (range, 1~4). There was no operative mortality or disease recurrence.
The resection of endophytic gastric SMT is difficult to approach and the large amount of normal gastric tissue is resected with gastric SMT unnecessarily in the extragastric approach. Especially when the tumor is located in the cardia, the operator must mobilize the retroperitoneal fixed point or use special devices with long arm or curvature. To overcome these limitations, the transgastric approach can be used. But in this approach, the operator have to open the stomach and the tumor should be took out. In the procedure, tumor cell can be disseminated in the peritoneal cavity. In the intragastric approach, the tumor located in the cardia or high lying posterior wall can be directly visualized, and easily manipulated. Also, the exposure of the tumor into the peritoneal cavity will be minimized.
Intragastric wedge resection using various techniques has been reported. In 2008, laparoscopic techniques using 4 ports (10 mm port for 10 mm intra abdominal laparoscopy, 5 mm for 5 mm intragastric laparoscopy, 5 mm for lifting up, 12 mm for endo-GIA) were introduced by Li et al.(12) In this study, another surgeon was necessary for intraoperative oesophagogastroduodenoscopy (OGD) and special port systems for retaining the stomach were also needed. The specimen was extracted through the 12 mm port site extending the incision, and extracorporeal suture of the stomach was done safely and quickly. Most of the intragastric techniques required OGD and the specimen was extracted orally with OGD.(15) We didn't need intraoperative OGD. There were three big differences between conventional intragastric approach and single incision intragastric approach. First, the operative time was decreased because of the one gastrostomy and the extracorporeal repair without any additional skin incision. Always, the gastrostomy site is vertically at the lower body of the stomach. And, it is possible to pull down the stomach to naval level. Second, the specimen can be delivered without endoscope. The umbilical skin incision was about 2 cm and the specimen can be took out easily. Last, in this approach, better cosmesis can be achieved. Also, creating a pneumoperitoneum was unnecessary. In our study, the operation time was 83.6 minutes but in most other reports, it was over 100 minutes.(12,15) The limitation of this procedure is the number of consumed stapler. The stapled resection with one stapler can be performed in the case of polypoid endophytic SMT. But in the most cases, the tumor was sessile and the whole nodule can be difficult to lift up without direct manipulation of the tumor, so multiple staplers were necessary. Usually three short staplers (45 mm Endo-GIA) should be needed. From the oncological point of view, local recurrence and distant metastasis including peritoneal seeding after the procedure are an index of safety. Usually GISTs are not infiltrating, and nodal metastasis is rare. Wedge resection with a negative margin is standard surgical procedure. In our report, the rate of recurrence was 0% in 8.5 months of follow-up period. Also, during the procedure, it is important that the tumor is not ruptured because of tumor dissemination in the peritoneal cavity. But in a single incision laparoscopic intragastric approach, though the tumor is ruptured during manipulation, dissemination of tumor cells into the peritoneal cavity may not occur.
Several intragastric wedge resection procedures for gastric SMT by laparoscopy have been reported. The feasibility and usefulness of intragastric resections of SMTs located near the esophagogastric junction or posterior gastric wall has been reported.(11) However, there are few reports about such operations using a single incision technique. Sasaki et al. reported an initial clinical experience with single incision laparoscopic access surgery (SILAS) for gastric submucosal tumors.(6) The mean operating time was 86 min and there was no morbidity and mortality. The results in this study were comparable with those in other conventional laparoscopic reports. But there were only 3 cases. SILAS may be beneficial for cosmesis, and for reducing immediate postoperative pain and invasiveness. Our single incision intragastric wedge resection was safe and technically feasible, with no recurrence. Also, the technique and equipment used in single incision surgery is not different from those used in conventional laparoscopic surgery. Although the technical challenges such as the limitation of the range of motion make it difficult, the learning curve will be overcome shortly with the development of instrument and port system.
Many reports recommend that intragastric wedge resection should be applied to a small sized and high lying, benign gastric tumor.(6,11,12,15) The size of tumor was below 5 cm, and an ulcer formation was absent. So like the intragastric approach was limited to the selected case, the single incision laparoscopic surgery of intragastric approach would be safe and feasible in selected patients.
In conclusion, single incision laparoscopic intragastric wedge resection is a feasible and safe procedure for high lying gastric submucosal lesions that require surgical resection. And direct visualization of the tumor, the minimization of the exposure of tumor in the peritoneal cavity, the ease of approach in the cardia and esophagogastric junction, decrease time of operation, and better cosmesis are the advantages of the intragastric single incision approach. But the number of cases reported was small. We now need prospective randomized studies. Also, technical difficulties during the single incision approach should be overcome. Development of laparoscopic equipment is also needed.
Figures and Tables
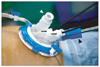
Fig. 1
Single port device (OCTO™ Dalim, Seoul, Korea) was placed at the umbilicus of patient. Th ere were 4 ports. A black arrow head (▴) is 5~12 mm transformable port. A white arrow head (▵) is 5/10 mm port with dual sealing mechanism. Two arrows (↑) are ports for 5 mm devices. In our procedure, the 5~12 mm port was for 10 mm telescope, 5/10 mm port was for 10 mm endo-GIA.
References
1. Kwon JG, Kim EY, Kim YS, Chun JW, Chung JT, You SS, et al. Accuracy of endoscopic ultrasonographic impression compared with pathologic diagnosis in gastrointestinal submucosal tumors. Korean J Gastroenterol. 2005. 45:88–96.
2. Wiech T, Walch A, Werner M. Histopathological classification of nonneoplastic and neoplastic gastrointestinal submucosal lesions. Endoscopy. 2005. 37:630–634.

3. Casali PG, Jost L, Reichardt P, Schlemmer M, Blay JY. ESMO Guidelines Working Group. Gastrointestinal stromal tumours: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009. 20:Suppl 4. 64–67.

4. Ryu KJ, Jung SR, Choi JS, Jang YJ, Kim JH, Park SS, et al. Laparoscopic resection of small gastric submucosal tumors. Surg Endosc. 2011. 25:271–277.

5. Blackstein ME, Blay JY, Corless C, Driman DK, Riddell R, Soulières D, et al. Canadian Advisory Committee on GIST. Gastrointestinal stromal tumours: consensus statement on diagnosis and treatment. Can J Gastroenterol. 2006. 20:157–163.

6. Sasaki A, Koeda K, Obuchi T, Nakajima J, Nishizuka S, Terashima M, et al. Tailored laparoscopic resection for suspected gastric gastrointestinal stromal tumors. Surgery. 2010. 147:516–520.

7. Matthews BD, Walsh RM, Kercher KW, Sing RF, Pratt BL, Answini GA, et al. Laparoscopic vs open resection of gastric stromal tumors. Surg Endosc. 2002. 16:803–807.

8. Novitsky YW, Kercher KW, Sing RF, Heniford BT. Long-term outcomes of laparoscopic resection of gastric gastrointestinal stromal tumors. Ann Surg. 2006. 243:738–745.

9. Sexton JA, Pierce RA, Halpin VJ, Eagon JC, Hawkins WG, Linehan DC, et al. Laparoscopic gastric resection for gastrointestinal stromal tumors. Surg Endosc. 2008. 22:2583–2587.

10. Lai IR, Lee WJ, Yu SC. Minimally invasive surgery for gastric stromal cell tumors: intermediate follow-up results. J Gastrointest Surg. 2006. 10:563–566.

11. Tagaya N, Mikami H, Kogure H, Kubota K, Hosoya Y, Nagai H. Laparoscopic intragastric stapled resection of gastric submucosal tumors located near the esophagogastric junction. Surg Endosc. 2002. 16:177–179.

12. Li VK, Hung WK, Chung CK, Ying MW, Lam BY, Kan DM, et al. Laparoscopic intragastric approach for stromal tumours located at the posterior gastric wall. Asian J Surg. 2008. 31:6–10.

13. Uchikoshi F, Ito T, Nishida T, Kitagawa T, Endo S, Matsuda H. Laparoscopic intragastric resection of gastric stromal tumor located at the esophago-cardiac junction. Surg Laparosc Endosc Percutan Tech. 2004. 14:1–4.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download