Abstract
Gastric necrosis due to gastric outlet obstruction is a very rare condition, but it might be fatal if missed or if diagnosis is delayed. Our patient was a 73-year-old male complaining of abdominal pain, distension and dyspnea for 1 day. In plain radiography and computed tomography, a markedly distended stomach and decreased enhancement at the gastric wall were noted. He underwent explo-laparotomy, and near-total gastric mucosal necrosis accompanied by sludge from the soaked laver was noted. A total gastrectomy with esophagojejunostomy was performed, and he recovered without sequelae. Final pathologic examination revealed advanced gastric cancer at the antrum with near-total gastric mucosal necrosis.
Gastric necrosis due to acute gastric dilation is a very rare clinical condition that may become fatal. Although gastric necrosis has been reported in patients with small-bowel obstruction, patients with bulimia and patients who cannot belch or vomit after Nissen fundoflication,(1-3) there was no report on near-total gastric necrosis resulting from gastric outlet obstruction due to sludge of the laver associated with advanced gastric cancer. Herein, we report our experience in patients with near-total gastric mucosal necrosis following large sludge of the soaked laver with advanced gastric cancer, which was successfully treated by total gastrectomy.
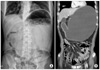
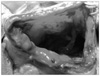
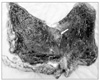
The patient was a 73-year-old male complaining of abdominal pain, distension and dyspnea for 1 day. He had suffered hemiplegia resulting from a cerebral infarction 10 years ago and had received anticoagulation agents since then. There was no other medical history, and he never underwent gastrofiberscopy. He had grazed the laver as a snack. On physical examination, the abdomen was severely distended and presented tenderness with muscle guarding at the middle abdomen. Blood pressure was 70/40 mmHg with a pulse rate of 130 beat/min. Laboratory investigations included hemoglobin 17.8 g/dl, white blood cell count 25,730/L, platelet count 293,000/L, blood urea nitrogen 21.6 mg/dl, creatinine 1.3 mg/dl, albumin 4.9 g/dl and prothrombin time of 14.5 sec. In a plain abdominal radiography, a marked distended stomach was identified with impacted food materials. Computed tomography revealed a massively dilated stomach with low enhancement at the gastric wall and an air bubble around the perigastric area. However, there was no definite lesion that resulted in gastric outlet obstruction (Fig. 1). Initially, a nasogastric tube was applied, but there was no drainage. Judging from the patient's status and radiologic findings, we suspected gastric necrosis due to the presence of gastric outlet obstruction and performed emergency laparotomy. Operative findings revealed that the stomach was massively distended and that a large sludge of the soaked laver was impacted at the antrum when gastrotomy was performed. After the removal of gastric contents, we examined the gastric mucosa. The entire gastric mucosa exhibited generalized edematous changes and easily came off with palpation (Fig. 2). We observed several enlarged lymph nodes around the perigastric area and mild hardness of the antrum; however, there were no obvious findings suggesting gastric cancer despite the severe gastric dilation. We suspected gastric cancer associated with gastric outlet obstruction. However, we did not perform a definitive cancer surgery because of the patient's condition. The patient underwent total gastrectomy with D1 lymph node dissection. Examination of the resected specimen revealed longitudinal dehiscence of the gastric wall at the lesser curvature side and near-total necrosis of the gastric mucosa (Fig. 3). Final pathologic examination revealed Borrman type-III gastric cancer with poorly differentiated adenocarcinoma located at the antrum. An 11×8.5 cm-sized tumor had invaded the serosal layer and extended to the pyloric ring. In total, 67 lymph nodes were recovered; among them, four lymph node metastases were observed. After the operation, the patient resumed an oral diet 4 days after surgery and was discharged at 17 days. There were no specific events, and the patient recovered. He did not receive adjuvant chemotherapy because of his general status and old age. However, he was readmitted because of pneumonia 4 months after the operation and died 7 days after that admission.
Acute gastric necrosis is a very rare clinical condition. The reported causes of gastric necrosis are various, including intrathoracic herniation of the stomach, volvulus, acute necrotizing gastritis, vascular compromise and acute gastric dilation.(4-7) Acute gastric dilation was first described by S.E. Duplay,(8) and diverse factors including postoperative adhesion, anorexia nervosa, trauma, and superior mesenteric artery syndrome were proven to be related. The pathogenesis for acute gastric distension and maximum capacity of the stomach has been studied in a cadaver model. Revilloid noted that maximal gastric capacity was 4 L and that perforation might occur if more than 4 L of anything is ingested.(5) Moreover, Kernstein reported that gastric capacity was about 15 L in bulimia patients.(1) It was known that intragastric pressure must exceed gastric venous pressure to result in ischemia(9) and that occlusion of both the artery and vein secondary to intragastric pressure was necessary to foster ischemia.(10) The stomach has a rich blood supply, and infarction did not occur even if four major gastric arteries were ligated. This supported the idea that arterial occlusion alone did not result in ischemia. There were some reports claiming that intragastric pressure can result in occlusion of the gastric luminal blood circulation.(1,9) Powell reported that pressure in the gastric lumen must be greater than 20 cmH2O, while Edlich reported a minimum value of 30 cmH2O. In review of our case, gastric cancer invading the pyloric ring result in forming a large bolus of the soaked laver and these conditions might be the cause of gastric necrosis following gastric outlet obstruction and dilation.
Clinically, more than 90% of patients complained of emesis.(11) Abdominal pain and dyspnea secondary to progressive abdominal distension, hypovolemia and oliguria might be presented. Upon physical examination, tympanic sound resulting from percussion on the abdomen and generalized muscle guarding in cases of perforation might be presented. Plain radiography revealed marked gastric distension with the air fluid level and pneumoperitoneum as well as subcutaneous emphysema if perforation resulted.(11,12)
The guiding principle of surgical therapy was the decompression of intragastric pressure. Initial therapy consisted of nasogastric tube insertion and fluid resuscitation. However, if conservative treatment could not improve the condition, prompt surgical intervention should be considered.(13) The critical point in surgical intervention was adequate resection of the necrotic portion of the stomach after identification of the status of the gastric mucosa with gastrotomy. Total gastrectomy with esophagojejunostomy or gastrectomy with cervical esophagostomy should be performed according to the patient's condition.(8) It was also insisted that a feeding jejunostomy should be performed(12); however, we did not perform this procedure due to the advancement of parenteral nutritional agents.
Early recognition was important; the mortality rate was 80% in cases when treatment was delayed.(12,14) Indeed, the mortality rate was 50~65% with surgical therapy and 100% without treatment.(13) We carried out prompt surgical therapy, and the patient recovered without specific events.
Gastric necrosis following acute gastric dilatation rarely occurs, but it can be fatal. Indeed, advanced gastric cancer at the distal antrum and a large bolus of soaked laver could result in gastric outlet obstruction; the surgeon should therefore keep this possibility in mind. It might be important to recognize this condition early and to determine the extent of the gastric resection by identifying the status of the gastric mucosa via gastrotomy during surgery.
Figures and Tables
Fig. 1
Finding of plain radiography (A) and computed tomography (B). Massive gastric distension and air bubble at perigastric area was shown.
References
1. Powell JL, Payne J, Meyer CL, Moncla PR. Gastric necrosis associated with acute gastric dilatation and small bowel obstruction. Gynecol Oncol. 2003. 90:200–203.

2. Glick PL, Harrison MR, Adzick NS, Webb HW, Delorimier AA. Gastric infarction secondary to small bowel obstruction: a preventable complication after Nissen fundoplication. J Pediatr Surg. 1987. 22:941–943.

3. Turan M, Sen M, Canbay E, Karadayi K, Yildiz E. Gastric necrosis and perforation caused by acute gastric dilatation: report of a case. Surg Today. 2003. 33:302–304.

5. Kerstein MD, Goldberg B, Panter B, Tilson MD, Spiro H. Gastric infarction. Gastroenterology. 1974. 67:1238–1239.

6. Strauss RJ, Friedman M, Platt N, Gassner W, Wise L. Gangrene of the stomach: a case of acute necrotizing gastritis. Am J Surg. 1978. 135:253–257.

7. Adachi Y, Takamatsu H, Noguchi H, Tahara H, Mukai M, Akiyama H. Spontaneous rupture of the stomach in preschool age children: a report of two cases. Surg Today. 1998. 28:79–82.

8. Todd SR, Marshall GT, Tyroch AH. Acute gastric dilatation revisited. Am Surg. 2000. 66:709–710.
9. Edlich RF, Borner JW, Kuphal J, Wangensteen OH. Gastric blood flow. I. Its distribution during gastric distention. Am J Surg. 1970. 120:35–37.
10. Babkin BP, Armour JC, Webster DR. Restoration of the functional capacity of the stomach when deprived of its main arterial blood supply. Can Med Assoc J. 1943. 48:1–10.
11. Chaun H. Massive gastric dilatation of uncertain etiology. Can Med Assoc J. 1969. 100:346–348.
12. Reeve T, Jackson B, Scott-conner C, Sledge C. Near-total gastric necrosis caused by acute gastric dilatation. South Med J. 1988. 81:515–517.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download