Abstract
Purpose
Currently, the two most influential gastric stem cell marker candidates are CD44 and CD133. The aim of this study was to make a comparison and determine the appropriate marker for use in gastric cancer stem cell research.
Materials and Methods
We analyzed the expressions of CD44, CD133, and CD24 from the gastric cancer cell lines MKN45, MKN74, KATO-III, NCI-N87, SNU-1, SNU-216, SNU-601, SNU-638, and SNU-688 using flow cytometry. In addition, we measured the change in viability after applying 5 fluorouracil (5-FU) to the MKN45, MKN74, KATO-III, and NCI-N87 cell lines using a Cell Counting Kit 8.
Results
CD133 expression was above moderate in the KATO-III, SNU-216, SNU-601 cell lines, whereas it was below 1% in the remaining cell lines. CD44 was expressed at levels above 5% in all gastric cancer cell lines. The effect of 5-FU on viability and CD133 or CD44 expression in the cell lines were not related.
Figures and Tables
Fig. 1
Expression of CD133, CD44, CD24 on gastric cancer cell lines using flow cytometry. (A) Expression of CD133 was detected only on KATO-III, SNU-216 and SNU-601. (B) Expressions of CD44 were above 5% on all cell lines. CD44+/CD24+ subpopulations were found to be highly expressive on MKN45 and SNU-688.

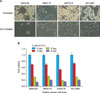
Fig. 2
Morphologic appearance and viability of 5-FU treated gastric cancer cell lines; KMN45, KMN74, KATO-III, NCI-N87. (A) Gastric cancer cells were incubated with RPMI and 5 µM of 5-FU. Cells were examined by light microscopy on the 8th day of treatment. (B) Cell viability measured by CCK-8 assays. 5 µM of 5-FU was added to gastric cancer cell lines and cell viability was measured using the CCK-8 assay on 0 day, 2 day, 5 day and 8 day. Cell viability was slightly more decreased in KATO-III cells than others.
References
1. Dick JE, Bhatia M, Gan O, Kapp U, Wang JC. Assay of human stem cells by repopulation of NOD/SCID mice. Stem Cells. 1997. 15:Suppl 1. 199–203.

2. Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003. 100:3983–3988.

3. Dick JE. Stem cells: self-renewal writ in blood. Nature. 2003. 423:231–233.
4. Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R, et al. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 2009. 27:1006–1020.

5. Huang P, Wang CY, Gou SM, Wu HS, Liu T, Xiong JX. Isolation and biological analysis of tumor stem cells from pancreatic adenocarcinoma. World J Gastroenterol. 2008. 14:3903–3907.

6. Li C, Lee CJ, Simeone DM. Identification of human pancreatic cancer stem cells. Methods Mol Biol. 2009. 568:161–173.

7. Ha TK, Kwon SJ. Clinicopathologic characteristics according to the type of recurrence in curtively-resected gastric cancer patients. J Korean Gastric Cancer Assoc. 2007. 7:23–30.

8. Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004. 432:396–401.

9. O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007. 445:106–110.
10. Suetsugu A, Nagaki M, Aoki H, Motohashi T, Kunisada T, Moriwaki H. Characterization of CD133+ hepatocellular carcinoma cells as cancer stem/progenitor cells. Biochem Biophys Res Commun. 2006. 351:820–824.

11. Rutella S, Bonanno G, Procoli A, Mariotti A, Corallo M, Pri sco MG, et al. Cells with characteristics of cancer stem/pro genitor cells express the CD133 antigen in human endometrial tumors. Clin Cancer Res. 2009. 15:4299–4311.

12. Suva ML, Riggi N, Stehle JC, Baumer K, Tercier S, Joseph JM, et al. Identification of cancer stem cells in Ewing's sarcoma. Cancer Res. 2009. 69:1776–1781.

13. Friedman S, Lu M, Schultz A, Thomas D, Lin RY. CD133+ anaplastic thyroid cancer cells initiate tumors in immunodeficient mice and are regulated by thyrotropin. PLoS One. 2009. 4:e5395.

14. Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008. 15:504–514.

15. Smith LM, Nesterova A, Ryan MC, Duniho S, Jonas M, Anderson M, et al. CD133/prominin-1 is a potential therapeutic target for antibody-drug conjugates in hepatocellular and gastric cancers. Br J Cancer. 2008. 99:100–109.

16. Boegl M, Prinz C. CD133 expression in different stages of gastric adenocarcinoma. Br J Cancer. 2009. 100:1365–1366.

17. LaBarge MA, Bissell MJ. Is CD133 a marker of metastatic colon cancer stem cells? J Clin Invest. 2008. 118:2021–2024.

18. Shmelkov SV, Butler JM, Hooper AT, Hormigo A, Kushner J, Milde T, et al. CD133 expression is not restricted to stem cells, and both CD133+ and CD133- metastatic colon cancer cells initiate tumors. J Clin Invest. 2008. 118:2111–2120.

19. Hsieh HF, Yu JC, Ho LI, Chiu SC, Harn HJ. Molecular studies into the role of CD44 variants in metastasis in gastric cancer. Mol Pathol. 1999. 52:25–28.

20. Yeung TM, Gandhi SC, Wilding JL, Muschel R, Bodmer WF. Cancer stem cells from colorectal cancer-derived cell lines. Proc Natl Acad Sci U S A. 2010. 107:3722–3727.

21. Hurt EM, Kawasaki BT, Klarmann GJ, Thomas SB, Farrar WL. CD44+ CD24(-) prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br J Cancer. 2008. 98:756–765.

22. Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008. 68:4311–4320.

23. Yook JH, Choi WY, Shin DG, Kim YJ, Kim JS, Oh ST, et al. Expression of E-cardherin and CD44H in bormann type IV gastric cancer. J Korean Gastric Cancer Assoc. 2004. 4:82–88.

24. Mirecka J, Marx D, Schauer A. Immunohistochemical localization of CD44 variants 5 and 6 in human gastric mucosa and gastric cancer. Anticancer Res. 1995. 15:1459–1465.
25. Ishimoto T, Oshima H, Oshima M, Kai K, Torii R, Masuko T, et al. CD44+ slow-cycling tumor cell expansion is triggered by cooperative actions of Wnt and prostaglandin E2 in gastric tumorigenesis. Cancer Sci. 2010. 101:673–678.

26. Nishii T, Yashiro M, Shinto O, Sawada T, Ohira M, Hirakawa K. Cancer stem cell-like SP cells have a high adhesion ability to the peritoneum in gastric carcinoma. Cancer Sci. 2009. 100:1397–1402.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download