Abstract
Ulcerative colitis (UC) is a chronic idiopathic inflammatory bowel disease. Mesalizine for the first-line therapy of UC has adverse effects include pancreatitis, pneumonia and pericarditis. UC complicated by two coexisting conditions, however, is very rare. Moreover, drug-related pulmonary toxicity is particularly rare. An 11-year-old male patient was hospitalized for recurring upper abdominal pain after meals with vomiting, hematochezia and exertional dyspnea developing at 2 weeks of mesalizine therapy for UC. The serum level of lipase was elevated. Chest X-ray and thorax computed tomography showed interstitial pneumonitis. Mesalizine was discontinued and steroid therapy was initiated. Five days after admission, symptoms were resolved and mesalizine was resumed after a drop in amylase and lipase level. Symptoms returned the following day, however, accompanied by increased the serum levels of amylase and lipase. Mesalizine was discontinued again and recurring symptoms rapidly improved.
Ulcerative colitis (UC) is a chronic idiopathic inflammatory bowel disease (IBD) that less common in Korea compared to the western societies. However, it is continuously becoming more prevalent in Korea due to recent changes in lifestyle and diet [1] and rapidly rising in the Korean pediatric population as well [2]. Inflammatory response as part of abnormal immune response is suspected to be a major pathophysiological mechanism of it [3]. As an anti-inflammatory agent, 5-Aminosalicylic acid (5-ASA) is widely used as the first-line therapy for acute flares and remission maintenance of UC.
Mesalizine, consisting of only 5-ASA molecules, is known to have excellent efficacy and safety, however, adverse effects such as headache, fever, eruption, nausea, diarrhea, pancreatitis, pneumonia, and pericarditis have been reported in some patients [4].
Along with literature review, we report a case of UC that was complicated by coexisting acute pancreatitis and interstitial pneumonitis during mesalizine therapy and improved rapidly after discontinuation.
An 11-year-old male patient presented with abdominal pain and hematochezia 2 weeks ago and was diagnosed as UC (pediatric UC activity index, PUCAI: 40) by colonoscopy with biopsy at Chosun University Hospital (Fig. 1). The patient was given mesalizine (Pentasa; 3,000 mg/day) for 2 weeks, and then symptoms were resolved. He was admitted for coughing and exertional dyspnea, however, as well as recurring upper abdominal pain after meals with vomiting and hematochezia developing 5 days before admission (PUCAI: 45). There is no smoking or alcohol drinking except taking mesalizine.
At admission, the body scale had body weight of 51 kg (50-75 percentile), height of 165 cm (90-95 percentile). He had blood pressure of 100/80 mmHg, pulse rate of 88/min, respiration rate of 18/min, body temperature of 36.3℃, and oxygen saturation of 99%. Chest auscultation findings during physical examination were normal, however, tenderness in the epigastrium and hyperactive bowel sound were observed.
Peripheral blood test results were as following; hemoglobin 13.8 g/dL, white blood cell 10,340/mm2 (neutrophil 69.4%, lymphocyte 18.4%, eosinophil 1.5%), platelet 514,000/mm2, erythrocyte sedimentation rate (ESR) 22 mm/hr (reference [ref.] 0-15 mm/hr) , and C-reactive protein (CRP) 6.49 mg/dL (ref. 0.0-0.3 mg/dL). Arterial blood gas analysis findings were pH 7.431, pCO2 35.0 mmHg, pO2 93.9 mmHg, HCO3 22.8 mmHg, and SaO2 97.4%. Biochemical examination showed elevated serum levels of lipase at 308 IU/L (ref. 13-60 IU/L). Serum level of triglyceride was 75 mg/dL (ref. 42-168 mg/dL) and no abnormalities were found on renal function test, liver function test or cardiac enzyme test. Fecal occult blood test was positive.
Chest X-ray revealed peripheral reticulonodular opacities in left lung field and thorax computed tomography (CT) showed nodular ground glass opacities in left lower lung zone periphery (Fig. 2). Pulmonary function test showed forced vital capacity (FVC) of 43% (1.56 L) and forced expiratory volume-one second (FEV1) of 42% (1.41 L) of predicted (FEV1/FVC 91 %), indicating severe restrictive changes (Fig. 3).
Enlarged pancreas or fluid collection around pancreas, suggestive of acute pancreatic, were not seen on abdominal CT and magnetic resonance cholangiopancreatography.
A day after admission, the patient developed a fever of 38.5℃, persistent abdominal pain and hematochezia, suggestive of worsening symptoms of UC. Mesalazine was discontinued and methylprednisolone was intravenously administered at 1 mg/kg/day. Typical therapy for acute pancreatitis was carried out including total parenteral nutrition and fasting. And also an inhalation bronchodilator (salbutamol) was concurrently given for dyspnea.
Five days after admission, fever, abdominal pain, and hematochezia were resolved. Serum amylase, lipase, ESR and CRP levels decreased to 22 IU/L, 49 IU/L, 15 mm/hr, and 0.24 mg/dL, respectively. Along with gradual diet, mesalizine was resumed for maintaining remission. However, fever and abdominal pain recurred the following day and amylase and lipase levels increased to 148 IU/L, and 325 IU/L, respectively. The patient continued to complain of exertional dyspnea after retry of mesalizine during his hospital days.
The hospital course showed that, in our opinion, mesalizine led to complications as pancreatitis and pneumonitis. Fasting was resumed and mesalizine was discontinued. Eleven days after admission, fever and abdominal symptoms were resolved and the amylase level dropped to 45 IU/L. Normal diet was resumed and methylprednisolone therapy was continued without recurrence of symptoms. Dyspnea perceived by the patient also improved after discontinuing mesalizine. Thorax CT showed rapid improvement of nodular ground glass opacities (Fig. 2 and 4).
Thirty days after admission, fever, abdominal pain and hematochezia were disappeared. Oral steroid was reduced and balsalazide was initiated to maintain remission. Currently, the patient is on balsalazide only and followed-up at the outpatient ward without special symptoms.
UC is recurrent IBD without clear etiology and its treatment consists of anti-inflammatory drugs such as sulfasalazine or 5-ASA and steroids. Immunosuppressants such as azathioprine, and 6-mercaptopurine can also be used in steroid-dependent or non-responsive patients. Various side effects of such drugs reported so far have included allergic responses such as fever, rash, nausea, and diarrhea, as well as infection, pancreatitis, eosinophilic pneumonia, and interstitial pneumonitis [56].
Mesalizine is a primary therapy for UC and consists of only 5-ASA molecules. It is reported to have good efficacy and safety profiles in about 80% of patients with hypersensitivity to sulfasalazine [7]; however, mesalizine-related side effects recently reported include pancreatitis, allergic lung reaction, and pericarditis [89].
On the other hand, lesions of UC are not limited to the intestines but are known to also affect other organs such as the eye, skin, and joints. Extraintestinal complications such as pancreatitis and pneumonia have also been extensively reported [10]. In case of such complications, it is important to distinguish UC-related complications from drug-induced complications.
Common causes of acute pancreatitis coexisting with UC include alcohol use, viral infection, cholelithiasis, hypertriglyceridemia, and other hepatobiliary disorders. When these causes are ruled out, drug-related side effects, extraintestinal manifestation of UC, or unknown causes can be suspected [11]. The pathophysiological mechanism of drug-related acute pancreatitis may involve increased permeability of the pancreatic duct due to exposure to high-dose 5-ASA and allergic reaction [12]. The incidence of drug-related pancreatitis is reported to increase 2-4 times in patients with IBD [13]. According to literature, pancreatitis develops within 2 days to 1 month of therapy and even 1 year after therapy in some cases [14]. However, there is no data supporting a clear correlation between the duration and dosage of medication and development of pancreatitis. Risk factors of UC-related acute pancreatitis are unclear but monitoring serum amylase in the first 1-2 months of therapy has been reported to lower the incidence of pancreatitis [15]. Most cases of 5-ASA-related pancreatitis developing during treatment of IBD have positive prognosis and are not fatal. High-dose steroid therapy can be considered in severe cases [12].
The exact mechanism of mesalizine-related pulmonary toxicity is not clear; however, oxidative damage due to the drug, direct alveolar capillary endothelial cell toxicity, damage from intracellular phospholipid deposits, and immunological damage may play a role [16]. Discontinuation of the causative drug is often followed by rapid improvement and good prognosis without sequalae or special findings on clinical and radiological examination. However, in cases with severe, persistent symptoms, steroids should be used [16].
Differentiating IBD-related pulmonary complications from drug-related pulmonary toxicity is very difficult. If complications develop in a patient in remission on continuous mesalizine therapy, drug induced conditions should be first suspected. Eosinophilic pneumonia, obstructive pulmonary disease, and interstitial pneumonia can commonly develop as a drug-induced complication [6] and when these conditions occur, the suspected causative drug should be discontinued and rechallenge test can be carried out to clarify the causal relationship with the drug.
UC complicated by two coexisting conditions, as in this case, is very rare. Moreover, drug-related pulmonary toxicity is particularly rare [17]. Acute pancreatitis and interstitial pneumonitis in this case was likely caused by therapy as rapid improvement in symptomatology and laboratory findings were seen after discontinuation but the same symptoms returned after resuming therapy. Moreover, extraintestinal manifestations of inflammation are known to follow the course of IBD [18]. Therefore, pancreatitis or pneumonitis developing in the remission phase after controlling the acute phase is likely a drug-induced complication.
Biologics such as infliximab are being tried in moderate to severe UC [19]. Biologics is useful as they can minimize side effects related to long-term steroid therapy and be applied in patients not responding to traditional therapy for UC. Biologics can be a good alternative in patients with hypersensitivity to medication and with contraindication for drugs other than steroids, as in the current case.
Therapeutic armamentarium for IBD is limited and most of the currently available choices can cause pancreatitis or pneumonitis. Accurate assessment of the activity of the underlying condition and history taking as well as identifying the relationship between timing of medication and development of clinical symptoms are important for preventing complications.
Figures and Tables
Fig. 1
Colonoscopy demonstrates diffuse symmetric erythematous mucosa with exudates from cecum to sigmoid colon, shallow ulcer at transverse colon.
Fig. 2
(A) Thorax computed tomography (CT) scan demonstrates nodular ground glass opacities in left lower lung zone periphery (arrow). (B) The lesion disappeared at follow-up CT scan 1 month later.
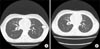
Fig. 3
Pulmonary function test revealed moderate to severe restriction (FVC 1.56 L [43% predicted], FEV1 1.41 L [42% predicted], and FEV1/FVC 91%). FVC: forced vital capacity, FEV1: forced expiratory volume-one second, PRE-RX: pre-response to bronchodilator, POST-RX: post-response to bronchodilator, %PRED: percentage of predicted value, %CHG: percentage of change.

Fig. 4
The clinical courses of the patient. Initially serum levels of amylase and lipase were 64 IU/L and 308 IU/L. Symptoms and biochemical results improved with NPO and discontinuation of mesalizine. However, a similar event recurred with the readministration of 5-aminosalicylic acid (5-ASA). With abdominal pain and fever (38.5℃), serum levels of amylase and lipase increased to 148 IU/L and 325 IU/L. Fever, dyspnea, and abdominal pain subsided and amylase levels returned to normal with NPO and discontinuation of mesalizine. HD: hospital date, PDS: prednisolone, NPO: non per oral intake.

References
1. Yang SK. Current status and clinical characteristics of inflammatory bowel disease in Korea. Korean J Gastroenterol. 2002; 40:1–14.
2. Seo JK, Yeon KM, Chi JG. Inflammatory bowel disease in children--clinical, endoscopic, radiologic and histopathologic investigation. J Korean Med Sci. 1992; 7:221–235.

3. Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007; 448:427–434.

4. Ransford RA, Langman MJ. Sulphasalazine and mesalazine: serious adverse reactions re-evaluated on the basis of suspected adverse reaction reports to the Committee on Safety of Medicines. Gut. 2002; 51:536–539.

5. Candy S, Wright J, Gerber M, Adams G, Gerig M, Goodman R. A controlled double blind study of azathioprine in the management of Crohn's disease. Gut. 1995; 37:674–678.

6. Williams T, Eidus L, Thomas P. Fibrosing alveolitis, bronchiolitis obliterans, and sulfasalazine therapy. Chest. 1982; 81:766–768.

7. Fardy JM, Lloyd DA, Reynolds RP. Adverse effects with oral 5-aminosalicyclic acid. J Clin Gastroenterol. 1988; 10:635–637.

8. Toubanakis C, Batziou E, Sipsas N, Galanopoulos G, Tzivras M, Archimandritis A. Acute pancreatitis after long-term therapy with mesalazine, and hyperamylasaemia associated with azathioprine in a patient with ulcerative colitis. Eur J Gastroenterol Hepatol. 2003; 15:933–934.

9. Woltsche M, Woltsche-Kahr I, Roeger GM, Aberer W, Popper H. Sulfasalazine-induced extrinsic allergic alveolitis in a patient with psoriatic arthritis. Eur J Med Res. 2001; 6:495–497.
10. Chikano S, Sawada K, Ohnishi K, Fukunaga K, Tanaka J, Shimoyama T. Interstitial pneumonia accompanying ulcerative colitis. Intern Med. 2001; 40:883–886.

11. Herrlinger KR, Stange EF. The pancreas and inflammatory bowel diseases. Int J Pancreatol. 2000; 27:171–179.

12. Fiorentini MT, Fracchia M, Galatola G, Barlotta A, de la Pierre M. Acute pancreatitis during oral 5-aminosalicylic acid therapy. Dig Dis Sci. 1990; 35:1180–1182.

13. Lankisch PG, Dröge M, Gottesleben F. Drug induced acute pancreatitis: incidence and severity. Gut. 1995; 37:565–567.

14. Balani AR, Grendell JH. Drug-induced pancreatitis : incidence, management and prevention. Drug Saf. 2008; 31:823–837.
15. Castiglione F, Del Vecchio Blanco G, Rispo A, Mazzacca G. Prevention of pancreatitis by weekly amylase assay in patients with Crohn's disease treated with azathioprine. Am J Gastroenterol. 2000; 95:2394–2395.

16. Foster RA, Zander DS, Mergo PJ, Valentine JF. Mesalamine-related lung disease: clinical, radiographic, and pathologic manifestations. Inflamm Bowel Dis. 2003; 9:308–315.

17. Camus P, Piard F, Ashcroft T, Gal AA, Colby TV. The lung in inflammatory bowel disease. Medicine (Baltimore). 1993; 72:151–183.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download