Abstract
Obesity is a state in which there is an over-accumulation of subcutaneous and/or abdominal adipose tissue. This adipose tissue is no longer considered inert and mainly devoted to storing energy; it is emerging as an active tissue in the regulation of physiological and pathological processes, including immunity and inflammation. Adipose tissue produces and releases a variety of adipokines (leptin, adiponectin, resistin, and visfatin), as well as pro- and anti-inflammatory cytokines (tumor necrosis factor-α, interleukin [IL]-4, IL-6, and others). Adipose tissue is also implicated in the development of chronic metabolic diseases such as type 2 diabetes mellitus or cardiovascular disease. Obesity is thus an underlying condition for inflammatory and metabolic diseases. Diet or dietary patterns play critical roles in obesity and other pathophysiological conditions. A healthy diet and some nutrients are generally considered beneficial; however, some dietary nutrients are still considered controversial. In this article, dietary factors that influence inflammation associated with obesity are discussed.
Obesity is a major global burden, at a worldwide cost of approximately $147 billion annually [1]. The World Health Organization (WHO) has reported that obesity has been growing at an alarming rate, accounting for approximately 35% of the population [2]. This proportion has consistently increased over the last decade. The Korean National Health and Nutrition Examination Survey (KNHANES) reported that the prevalence of obesity in Korea (defined as a body mass index [BM])≥25 kg/m2 according to WHO Asia Pacific guidelines) in men and women was 35.1% and 27.1%, respectively, in 2011 [3].
Obesity is complex chronic disorder with a multifactorial etiology, involving genetics, hormones, diets, and environments. In particular, the major factor contributing to obesity in Korea could be rapid changes in diet (a dramatic increase in the consumption of animal foods has occurred over a short period of time in Korea, 7.7 g%, in 1970 vs. 20.3 g%, in 2010) [4]. This phenomenon accompanied by the substantial economic growth and the concurrent adoption to a westernized lifestyle.
Adipose tissue, the main feature of obesity, was considered as an inert tissue mainly devoted to energy storage; however, it is now recognized as an active tissue in the regulation of physiological and pathological processes, including immunity and inflammation. Adipose tissue produces and releases a variety of adipokines and cytokines (anti- or pro-inflammatory cytokines), including the leptin, adiponectin, resistin, and visfatin, as well as interleukin [IL]-4, interferon [IFN]-γ, tumor necrosis factor [TNF]-α, IL-6, and others [5,6]. Pro-inflammatory molecules produced by adipose tissue have been implicated as active participants in the development of metabolic disease such as type 2 diabetes mellitus and cardiovascular disease (CVD) [7].
Previous studies have reviewed [8-10] the role of diet (e.g., calorie-restricted, vegetable and fruit rich, Mediterranean) and dietary factors (e.g., fatty acids, antioxidant nutrients) on inflammatory state and its relationship to obesity. Three major aspects of previous findings will be reviewed in this article. First, we consider adipose tissue as a major source of bioactive substances related to obesity and inflammation. Second, we examine obesity as a condition of chronic low-grade inflammation. Finally, we consider dietary factors that affect inflammation related to obesity.
Over the last decades, adipose tissue has been identified as a metabolically dynamic endocrine organ and an important source of several hormones, cytokines, chemokines, growth factors, and complement proteins [6,7]. These substances play a central role in whole body homeostasis by influencing a variety of biological and physiological processes (Table 1). They are control of food intake, energy balance, insulin action, lipid and glucose metabolism, angiogenesis and vascular remodeling, blood pressure, and coagulation.
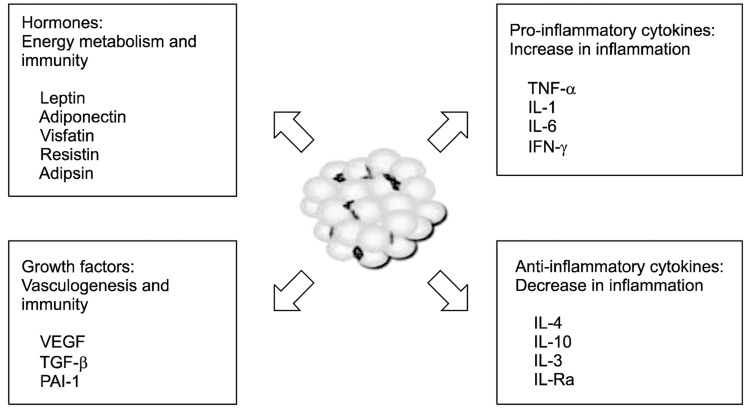
Adipose tissue has also been recognized as a heterogeneous tissue composed of several cell types: mature adipocytes, pre-adipocyte, fibroblasts, endothelial cells, mast cells, granulocytes, lymphocytes, and macrophages [11]. When adipocytes increase in number (hyperplasia) and size (hypertrophy), a various cytokines are secreted and contribute to the inflammatory process [12]. The representative adipokines and cytokines are as follows (Fig. 1):
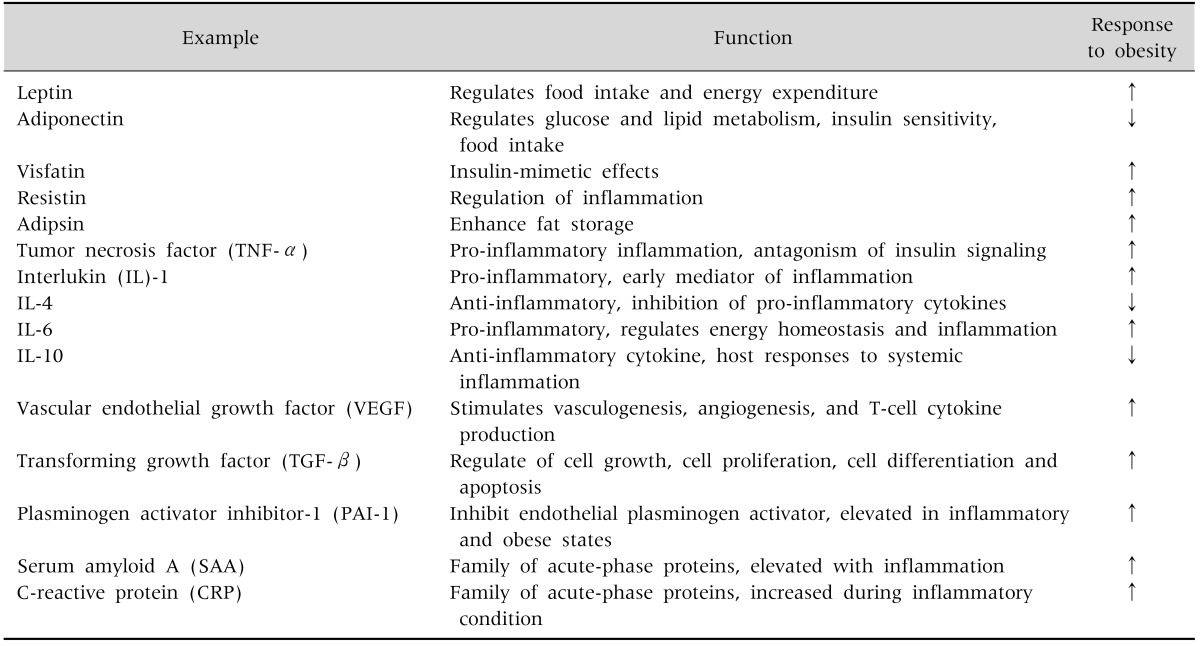
Leptin regulates food intake and energy expenditure [13]. It also regulates inflammatory responses, including the T-cell immune response, proliferation of T-helper cells, and the production of pro-inflammatory cytokines.
Adiponectin regulates glucose and lipid metabolism, insulin sensitivity, and food intake. It also protects against chronic inflammation [14].
Visfatin is a recently discovered adipokine produced and secreted primarily by visceral adipose tissue (VAT). Visfatin exerts insulin-mimetic effects via binding and activating the insulin receptor [15].
Resistin received its name for its role in inducing insulin resistance [16]. It is also known as a member of a family of molecules found in inflammatory zone (FIZZ) implicated in the regulation of inflammation.
Adipocyte trypsin (adipsin) is known to enhance fat storage in adipose tissues through the stimulation of glucose transport and fatty-acid re-esterification as well as the inhibition of lipolysis [17].
TNF-α is a pro-inflammatory cytokine predominantly synthesized by macrophages in adipose tissue [18].
IL-1 is a prototypical inflammatory cytokine and an early mediator of inflammation [19].
IL-6 is a pro-inflammatory cytokine that regulates energy homeostasis and inflammation [20].
IL-4 is an anti-inflammatory cytokine, and has marked inhibitory effects on the expression and release of pro-inflammatory cytokines [21].
IL-10 is the most important anti-inflammatory cytokine in the immune response. It has a physiological effect on host responses to systemic inflammation [22].
IFN-γ mediates both pro- and anti-inflammation. It regulates systemic inflammation and insulin resistance [23].
Obesity is associated with alterations in immunity, a chronic low-grade inflammation in which there are elevated circulating pro-inflammatory cytokines. However, it is unclear how obesity precisely triggers inflammation. Several hypotheses have been proposed. One hypothesis is that the overload of nutrients in adipocytes induces intracellular stress, resulting in the activation of inflammatory cascades [24,25]. The excessive nutrients may cause the accumulation of misfolded and/or unfolded proteins in the endoplasmic reticulum (ER), which activates the unfolded protein response (UPR) pathway [25]. The UPR pathway essentially depends on three main ER sensors; a PKR-like eukaryotic initiation factor 2α kinase (PERK), inositol-requiring enzyme 1 (IRE-1), and activating transcription factor 6 (ATF-6) [26]. These activated sensors could increase the activity of the C-Jun amino-terminal kinase (JNK) and inhibitor of IκB (IKK-β), serine-phosphorylation of insulin-receptor substrate protein 1 (IRS-1), and the nuclear factor κB (NFκB) pathway, leading to the enhanced expression of pro-inflammatory cytokines [24,27-29].
Second hypothesis suggests that the overloading of adipocytes with fat overwhelmingly increases the infiltration of macrophages. These processes may cause the subsequent differentiation and activation of cytotoxic T cells, which initiate and propagate inflammatory cascades [30].
Third hypothesis suggests that as adipose tissues enlarge, tissues become relatively hypoxic. Hypoxia within adipose tissue may activate inflammatory pathways [31,32].
The last hypothesis is that overloaded adipocytes can themselves directly activate immune pathogen-sensors that cause chronic inflammation [33].
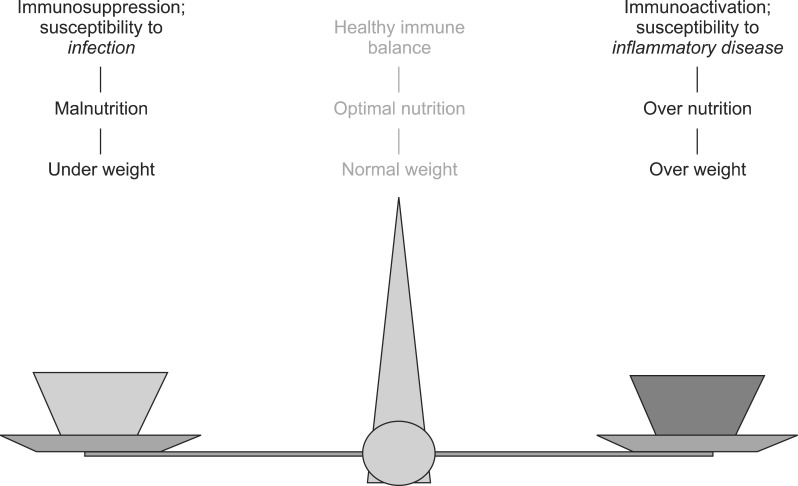
Diet is an important regulatory factor on immune response. There is considerable evidence to suggest that malnutrition leads to immunosuppression due to a susceptibility to infection. On the other hand, over-nutrition leads to immunoactivation due to a susceptibility to an inflammatory condition. Therefore, an optimal nutrition is required for a healthy immune balance (Fig. 2).
Carbohydrates are a main dietary energy source and can be evaluated according to glycemic index (GI) and glycemic load (GL) values. GI is a ranking of foods based on their postprandial blood glucose responses and a measure of carbohydrate quality [34]. GL is a measure that incorporates both the quantity and quality of dietary carbohydrates [35]. Large cross-sectional studies have shown an association between dietary GI/GL and inflammatory cytokines [36-39]. In the Women's Health Study (n=13,187, >45 years of age), the high quintiles of dietary GI and GL were significantly related to high blood levels of C-reactive protein (CRP) [36]. Similarly, in a Dutch study (n=974, 42-87 years of age), the highest quintile of dietary GI and GL was positively associated with blood levels of CRP (p<0.05) [37]. In addition, the Nurse Health Study (n=902 , 30-55 years of age) [38] and Health Professionals' Follow-Up Study (n=532, 40-75 years of age) [39] showed that a high dietary GI or GL was significantly associated with low plasma levels of adiponectin (p<0.05). The blood levels of high CRP and low adiponectin are characterized as low grade inflammatory status related with obesity. Interestingly, randomized clinical trials have not shown a relationship between a high GI or GL diet with inflammatory cytokines (CRP, TNF-α, and IL-6) [40,41]. However, 30% of energy restriction in the high GL showed a decline in serum CRP concentration in the healthy overweight adults (n=34, 24-42 years of age) [42]. Other large observational studies have shown a positive association between a high GI/GL diet and inflammatory markers; however, intervention studies could not convincingly support this association.
High fat diet causes excessive body fat accumulation and impairs the immune system. A number of different fatty acids, including polyunsaturated (PUFA), saturated, and trans-fatty acids have been studied for their effects on inflammatory status. Recently, Joffe et al. [43] reviewed that impact of dietary fatty acids on the gene expression of and production of TNFα and IL-6.
The omega-6 (n-6) PUFA and omega-3 (n-3) PUFA families are precursors of eicosanoids, which play an important role in the immune response. The anti-inflammatory effects of n-3 PUFA (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) have been observed in cross-sectional studies [44,45]. The Nurses' Health Study I (n=727, 43-69 years of age) [44] and the Attica Study (n=3,042, 18-89 years of age) [45] showed that the intake of n-3 fatty acids or fish is inversely associated with biomarkers of inflammation (CRP, IL-6, and TNF-α) (p<0.05). An interventional study (n=30, mean age of 60 years) also report that fish oil supplementation (14 g/d of fish oil for 5 weeks) decreased the blood levels of CRP and IL-6 in healthy postmenopausal women [46].
Observational and interventional studies suggest that trans- or saturated FAs are significantly related to the immune response. According to the Nurses' Health Study I Cohort (n=730, 43-69 years of age) [47] the highest quintile of trans-FAs consumption was associated with high levels of CRP and IL-6 compared with the lowest quintile. In a randomized cross-over study (n=50), the replacement of trans-FAs (8%) into a high-fat diet (39% of fat) significantly increased blood levels of CRP and IL-6 (p<0.05) [48]. Similarly, the substitution of trans-FAs (<7%) into a standard diet (30% of fat) increased IL-6 levels (as well as TNF-α levels but not CRP levels) in subjects with moderate hypercholesterolemia (18 men and 18 women; age range: females, 57-73 years of age; males, 52-73 years of age) [49].
Many cross-sectional studies and some observational studies [50-53] have reported an inverse association between a high amount of vegetables and fruits consumption, either in combination or alone, and CRP levels. The Boston Puerto Rican Health Study (n=1,159, 45-75 years of age) [50] also showed that variable fruit consumption was inversely correlated with blood levels of CRP. In contrast, studies by Salas-Salvadó et al. [51] (n=772, 55-80 years of age) and Freese et al. [52] (n=77, 19-52 years of age) did not show any association between a diet rich in vegetables and fruits with inflammatory markers (adiponectin, CRP, IL-6, intercellular adhesion molecule-1 [ICAM-1], vascular cell adhesion molecule-1 [VCAM-1]). Morand et al. [53] (n=24, mean age of 56 years) showed that a single fruit supplementation (500 mL of orange juice/d for 4 weeks) did not change the levels of CRP, IL-6, ICAM-1, and VCAM-1.
Some vitamins and minerals have been shown to have a beneficial effect on oxidative stress and immune responses. Cross-sectional and interventional studies [54-61] have consistently demonstrated that vitamins and minerals are associated with levels of inflammatory markers (CRP, TNF-α, and IL-6).
Overweight or obese subjects frequently reported that they have lower circulating carotenoids in plasma because of a high proportion of carotenoids, as lipid-soluble compounds, being stored in adipose tissue [54]. The Women's Health Study (n=2,895, aged ≥45 years of age) reported that higher plasma concentrations of α- and β-carotene were associated with low levels of plasma CRP [55].
Generally vitamin C has beneficial effects on immunity. Aasheim et al. [56] showed the low levels of plasma vitamin C were significantly associated with elevated levels of CRP in the subjects with morbid obesity (62 men and 106 women, 19-59 years of age). Block et al. [57] (n=216) supplemented vitamin C (515 mg/d for 8 weeks) to the healthy smokers. They found that supplementation of vitamin C significantly reduced blood levels of CRP (24%, p<0.05). On the other hand, Fumeron et al. [58] (n=42, 18-80 years of age) reported that vitamin C supplementation (750 mg/d for 8 weeks) did not changed blood levels of CRP.
In the Women's Health Study (n=3,713, 50-79 years of age), Mg intake was inversely associated with CRP, IL-6, and TNF-α-R2 levels in a dose-dependent manner after an adjustment for multiple variables, including dietary fiber, fat, fruits, and vegetables [59]. Guerrero-Romero and Rodríguez-Morán [60] showed that low serum Mg levels were independently related to elevated CRP concentration, in non-diabetic, non-hypertensive obese subjects (n=371).
Flavonoids, a subclass of biological polyphenolic compounds, are present in plant-derived vegetables and fruits, herbs, chocolate, tea, and red wine. Many epidemiology and intervention studies [61-65] have shown the clear antioxidant properties of flavonoids; however their inflammatory and immunoregulatory effects are less clear. The Nurses' Health Study (n=2,115, 43-70 years of age) showed that a diet rich in flavonoids (flavones, flavanones, and total flavonoids) was associated with low concentrations of the pro-inflammatory biomarkers (IL-18 and sVCAM-1) [61]. Recently, the United States Department of Agriculture (USDA, 2006) (n=8,335, ≥19 years of age) reported that a high consumption of dietary flavonoids was inversely associated with plasma CRP concentration (p<0.05) [62]. A randomized, controlled parallel study (n=120, 40-74 years of age) showed that supplementation of bilberry juice (300 mL/d for 3 weeks) reduced plasma levels of pro-inflammatory cytokines (TNF-α, IL-6, and IL-8, no changes in CRP) [63]. Recently, Karlsen et al. [64] reported that supplementation of bilberry juice (330 mL/d for 4 weeks) significantly reduced plasma levels of pro-inflammatory cytokines (TNF-α, IL-6, and IL-15) as well as CRP in the subjects who have CVD risks (n=62, 34-68 years of age). In contrast, a double-blind, placebo-controlled crossover study (n=14, 35-53 years of age) showed that the supplementation of sea buckthorn flavonol extract for 4 weeks did not reduced CRP levels (p<0.05) [65].
Phytoestrogens are plant-derived compounds found in a wide variety of foods, beans, seeds, and grains. Phytoestrogens are recognized to have anti-inflammatory properties. In a randomized, controlled study [66-69], a diet of pasta naturally enriched with isoflavone, aglycones (33 mg/d) significantly reduced plasma CRP concentrations. Plasma CRP levels returned to baseline when the subjects were switched back to a conventional diet (n=62, mean age of 58.2 years) [66]. Meanwhile, in a study of healthy postmenopausal women, the supplementation of soy isoflavone (genistein at 54 or 40 mg/d) for 6 months did not affect CRP concentrations (n=30, 50-60 years of age and n=80, mean age of 49.5 years) [67,68]. Similarly, in a study of obese postmenopausal women, soy isoflavone supplementation for 6 months had not effects on plasma CRP concentration (n=50, mean age of 58 years) [69]. There are some evidences of beneficial effects of phytoestrogen supplementation on inflammatory markers; however, the results are inconsistent.
Probiotics are living micro-organisms that have a health benefit for their host [70,71]. Orally ingested probiotic bacteria are able to modulate the immune system; however, differences exist in the immunomodulatory effects of different probiotic strains. In a randomized, double-blind, placebo-controlled trial, the combination of Lactobacillus gasseri and Lactobacillus coryniformis with Staphylococcus thermophilus for 2 or 4 weeks had no effect on serum TNF-α or IL-12 concentrations in healthy subjects (n=30, 23-43 years of age) [72,73]. Kekkonen et al. [74] compared L. rhamnosus with Bifidobacterium animalis ssp. lactis Bb12 and Propionibacterium freudenreichii ssp. Shermanii JS for 3 weeks in healthy subjects (n=81, 23-58 years of age). There was no effect on serum levels of TNF-α, IL-6, IL-10 or IFN-γ, but a decreased level of CRP in the L. rhamnosus supplementation group. Although the probiotics shown to have beneficial effects on inflammatory markers, further studies are required to reach the conclusive results.
Prebiotics are non-digestible food components that have a health benefit on the host that is associated with the modulation of microbiota in the gut [75]. Oligofructose, a typical type of prebiotics, supplementation (8 g/d for 3 weeks) in the elderly (n=19, mean age of 85 years) decreased the expression of IL-6 mRNA [76]. In contrast, oligofructose supplementation (1.95-3.9 g/d for 12 weeks) had no effect on plasma levels of IL-6 or TNF-α in poorly nourished elderly subjects (mean age of 70 years) [77]. Few observational studies [76,77] have shown a convincing association between a prebiotic supplementation and inflammatory markers however it is premature to draw this beneficial association at present.
Studies have conclusively shown that adipose tissue is an endocrine organ that plays a critical role on the homeostasis of immunity. An overload of nutrients induces obesity which is a state of a chronic low-grade inflammation. An optimal nutrition is thus plays an essential role in immunity. A healthy or prudential diet with appropriate GI/GL, n-3 PUFAs, vitamins, minerals, flavonoids, and with low amounts of trans- and saturated FAs is beneficial. The interaction of diet/nutrients with inflammation as well as the role of gene polymorphisms on inflammatory markers requires much greater exploration.
References
1. Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood). 2009; 28:w822–w831. PMID: 19635784.

2. World Health Organization. Obesity [Internet]. Geneva: WHO;2008. cited 2009 Oct 22. Available from: http://www.who.int/topics/obesity/en/.
3. Statistics Korea. Estimated future population 2010-2060. Daejeon: Statistics Korea;2011. cited 2011 Dec 7. Available from: http://kosis.kr/statisticsList/statisticsList_01List.jsp?vwcd=MT_ZTITLE&parentId=D.
4. Korea Centers for Disease Control and Prevention. The Korean National Health and Nutrition Examination Survey (KNHANES). 2011. Available at http://knhanes.cdc.go.kr/knhanes/.
5. Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004; 92:347–355. PMID: 15469638.

6. Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010; 316:129–139. PMID: 19723556.

7. Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008; 29:2959–2971. PMID: 18775919.

9. Bulló M, Casas-Agustench P, Amigó-Correig P, Aranceta J, Salas-Salvadó J. Inflammation, obesity and comorbidities: the role of diet. Public Health Nutr. 2007; 10:1164–1172. PMID: 17903326.

10. Calder PC, Ahluwalia N, Brouns F, Buetler T, Clement K, Cunningham K, et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr. 2011; 106(Suppl 3):S5–S78. PMID: 22133051.

11. Calabro P, Yeh ET. Obesity, inflammation, and vascular disease: the role of the adipose tissue as an endocrine organ. Subcell Biochem. 2007; 42:63–91. PMID: 17612046.
12. Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006; 6:772–783. PMID: 16998510.

13. Ceddia RB, Koistinen HA, Zierath JR, Sweeney G. Analysis of paradoxical observations on the association between leptin and insulin resistance. FASEB J. 2002; 16:1163–1176. PMID: 12153984.

14. Ekmekci H, Ekmekci OB. The role of adiponectin in atherosclerosis and thrombosis. Clin Appl Thromb Hemost. 2006; 12:163–168. PMID: 16708117.

15. Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005; 307:426–430. PMID: 15604363.

16. Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001; 409:307–312. PMID: 11201732.

17. Purohit A, Ghilchik MW, Duncan L, Wang DY, Singh A, Walker MM, et al. Aromatase activity and interleukin-6 production by normal and malignant breast tissues. J Clin Endocrinol Metab. 1995; 80:3052–3058. PMID: 7559896.

18. Koh KK, Han SH, Quon MJ. Inflammatory markers and the metabolic syndrome: insights from therapeutic interventions. J Am Coll Cardiol. 2005; 46:1978–1985. PMID: 16325028.
19. Mandrup-Poulsen T. The role of interleukin-1 in the pathogenesis of IDDM. Diabetologia. 1996; 39:1005–1029. PMID: 8877284.

20. Horn F, Henze C, Heidrich K. Interleukin-6 signal transduction and lymphocyte function. Immunobiology. 2000; 202:151–167. PMID: 10993289.

21. Brown MA, Hural J. Functions of IL-4 and control of its expression. Crit Rev Immunol. 1997; 17:1–32. PMID: 9034722.

22. Marchant A, Devière J, Byl B, De Groote D, Vincent JL, Goldman M. Interleukin-10 production during septicaemia. Lancet. 1994; 343:707–708. PMID: 7907683.

23. Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004; 75:163–189. PMID: 14525967.
24. Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011; 29:415–445. PMID: 21219177.

25. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006; 444:860–867. PMID: 17167474.

26. Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007; 8:519–529. PMID: 17565364.

27. Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000; 287:664–666. PMID: 10650002.

28. Deng J, Lu PD, Zhang Y, Scheuner D, Kaufman RJ, Sonenberg N, et al. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol Cell Biol. 2004; 24:10161–10168. PMID: 15542827.

29. Gregor MF, Hotamisligil GS. Thematic review series: adipocyte biology. Adipocyte stress: the endoplasmic reticulum and metabolic disease. J Lipid Res. 2007; 48:1905–1914. PMID: 17699733.

30. Surmi BK, Hasty AH. Macrophage infiltration into adipose tissue: initiation, propagation and remodeling. Future Lipidol. 2008; 3:545–556. PMID: 18978945.

31. Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007; 56:901–911. PMID: 17395738.

32. Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab. 2007; 293:E1118–E1128. PMID: 17666485.
33. Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006; 116:3015–3025. PMID: 17053832.

34. Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981; 34:362–366. PMID: 6259925.

35. Salmerón J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA. 1997; 277:472–477. PMID: 9020271.

36. Levitan EB, Cook NR, Stampfer MJ, Ridker PM, Rexrode KM, Buring JE, et al. Dietary glycemic index, dietary glycemic load, blood lipids, and C-reactive protein. Metabolism. 2008; 57:437–443. PMID: 18249220.

37. Du H, van der A DL, van Bakel MM, van der Kallen CJ, Blaak EE, van Greevenbroek MM, et al. Glycemic index and glycemic load in relation to food and nutrient intake and metabolic risk factors in a Dutch population. Am J Clin Nutr. 2008; 87:655–661. PMID: 18326604.

38. Qi L, Meigs JB, Liu S, Manson JE, Mantzoros C, Hu FB. Dietary fibers and glycemic load, obesity, and plasma adiponectin levels in women with type 2 diabetes. Diabetes Care. 2006; 29:1501–1505. PMID: 16801569.

39. Pischon T, Girman CJ, Rifai N, Hotamisligil GS, Rimm EB. Association between dietary factors and plasma adiponectin concentrations in men. Am J Clin Nutr. 2005; 81:780–786. PMID: 15817852.

40. Pittas AG, Roberts SB, Das SK, Gilhooly CH, Saltzman E, Golden J, et al. The effects of the dietary glycemic load on type 2 diabetes risk factors during weight loss. Obesity (Silver Spring). 2006; 14:2200–2209. PMID: 17189547.

41. Shikany JM, Phadke RP, Redden DT, Gower BA. Effects of low- and high-glycemic index/glycemic load diets on coronary heart disease risk factors in overweight/obese men. Metabolism. 2009; 58:1793–1801. PMID: 19631353.

42. Vrolix R, Mensink RP. Effects of glycemic load on metabolic risk markers in subjects at increased risk of developing metabolic syndrome. Am J Clin Nutr. 2010; 92:366–374. PMID: 20504977.

43. Joffe YT, Collins M, Goedecke JH. The relationship between dietary fatty acids and inflammatory genes on the obese phenotype and serum lipids. Nutrients. 2013; 5:1672–1705. PMID: 23698162.

44. Lopez-Garcia E, Schulze MB, Manson JE, Meigs JB, Albert CM, Rifai N, et al. Consumption of (n-3) fatty acids is related to plasma biomarkers of inflammation and endothelial activation in women. J Nutr. 2004; 134:1806–1811. PMID: 15226473.

45. Zampelas A, Panagiotakos DB, Pitsavos C, Das UN, Chrysohoou C, Skoumas Y, et al. Fish consumption among healthy adults is associated with decreased levels of inflammatory markers related to cardiovascular disease: the ATTICA study. J Am Coll Cardiol. 2005; 46:120–124. PMID: 15992645.

46. Ciubotaru I, Lee YS, Wander RC. Dietary fish oil decreases C-reactive protein, interleukin-6, and triacylglycerol to HDL-cholesterol ratio in postmenopausal women on HRT. J Nutr Biochem. 2003; 14:513–521. PMID: 14505813.

47. Lopez-Garcia E, Schulze MB, Meigs JB, Manson JE, Rifai N, Stampfer MJ, et al. Consumption of trans fatty acids is related to plasma biomarkers of inflammation and endothelial dysfunction. J Nutr. 2005; 135:562–566. PMID: 15735094.

48. Baer DJ, Judd JT, Clevidence BA, Tracy RP. Dietary fatty acids affect plasma markers of inflammation in healthy men fed controlled diets: a randomized crossover study. Am J Clin Nutr. 2004; 79:969–973. PMID: 15159225.

49. Lichtenstein AH, Erkkilä AT, Lamarche B, Schwab US, Jalbert SM, Ausman LM. Influence of hydrogenated fat and butter on CVD risk factors: remnant-like particles, glucose and insulin, blood pressure and C-reactive protein. Atherosclerosis. 2003; 171:97–107. PMID: 14642411.

50. Bhupathiraju SN, Tucker KL. Greater variety in fruit and vegetable intake is associated with lower inflammation in Puerto Rican adults. Am J Clin Nutr. 2011; 93:37–46. PMID: 21068354.

51. Salas-Salvadó J, Garcia-Arellano A, Estruch R, Marquez-Sandoval F, Corella D, Fiol M, et al. Components of the Mediterranean-type food pattern and serum inflammatory markers among patients at high risk for cardiovascular disease. Eur J Clin Nutr. 2008; 62:651–659. PMID: 17440519.

52. Freese R, Vaarala O, Turpeinen AM, Mutanen M. No difference in platelet activation or inflammation markers after diets rich or poor in vegetables, berries and apple in healthy subjects. Eur J Nutr. 2004; 43:175–182. PMID: 15168040.

53. Morand C, Dubray C, Milenkovic D, Lioger D, Martin JF, Scalbert A, et al. Hesperidin contributes to the vascular protective effects of orange juice: a randomized crossover study in healthy volunteers. Am J Clin Nutr. 2011; 93:73–80. PMID: 21068346.

54. Brady WE, Mares-Perlman JA, Bowen P, Stacewicz-Sapuntzakis M. Human serum carotenoid concentrations are related to physiologic and lifestyle factors. J Nutr. 1996; 126:129–137. PMID: 8558292.

55. Wang L, Gaziano JM, Norkus EP, Buring JE, Sesso HD. Associations of plasma carotenoids with risk factors and biomarkers related to cardiovascular disease in middle-aged and older women. Am J Clin Nutr. 2008; 88:747–754. PMID: 18779292.

56. Aasheim ET, Hofsø D, Hjelmesaeth J, Birkeland KI, Bøhmer T. Vitamin status in morbidly obese patients: a cross-sectional study. Am J Clin Nutr. 2008; 87:362–369. PMID: 18258626.

57. Block G, Jensen C, Dietrich M, Norkus EP, Hudes M, Packer L. Plasma C-reactive protein concentrations in active and passive smokers: influence of antioxidant supplementation. J Am Coll Nutr. 2004; 23:141–147. PMID: 15047680.

58. Fumeron C, Nguyen-Khoa T, Saltiel C, Kebede M, Buisson C, Drüeke TB, et al. Effects of oral vitamin C supplementation on oxidative stress and inflammation status in haemodialysis patients. Nephrol Dial Transplant. 2005; 20:1874–1879. PMID: 15972322.

59. Chacko SA, Song Y, Nathan L, Tinker L, de Boer IH, Tylavsky F, et al. Relations of dietary magnesium intake to biomarkers of inflammation and endothelial dysfunction in an ethnically diverse cohort of postmenopausal women. Diabetes Care. 2010; 33:304–310. PMID: 19903755.

60. Guerrero-Romero F, Rodríguez-Morán M. Relationship between serum magnesium levels and C-reactive protein concentration, in non-diabetic, non-hypertensive obese subjects. Int J Obes Relat Metab Disord. 2002; 26:469–474. PMID: 12075573.

61. Landberg R, Sun Q, Rimm EB, Cassidy A, Scalbert A, Mantzoros CS, et al. Selected dietary flavonoids are associated with markers of inflammation and endothelial dysfunction in U.S. women. J Nutr. 2011; 141:618–625. PMID: 21325476.

62. Chun OK, Chung SJ, Claycombe KJ, Song WO. Serum C-reactive protein concentrations are inversely associated with dietary flavonoid intake in U.S. adults. J Nutr. 2008; 138:753–760. PMID: 18356331.

63. Karlsen A, Retterstøl L, Laake P, Paur I, Bøhn SK, Sandvik L, et al. Anthocyanins inhibit nuclear factor-kappaB activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults. J Nutr. 2007; 137:1951–1954. PMID: 17634269.
64. Karlsen A, Paur I, Bøhn SK, Sakhi AK, Borge GI, Serafini M, et al. Bilberry juice modulates plasma concentration of NF-kappaB related inflammatory markers in subjects at increased risk of CVD. Eur J Nutr. 2010; 49:345–355. PMID: 20119859.
65. Suomela JP, Ahotupa M, Yang B, Vasankari T, Kallio H. Absorption of flavonols derived from sea buckthorn (Hippophaë rhamnoides L.) and their effect on emerging risk factors for cardiovascular disease in humans. J Agric Food Chem. 2006; 54:7364–7369. PMID: 16968106.

66. D'Anna R, Baviera G, Corrado F, Cancellieri F, Crisafulli A, Squadrito F. The effect of the phytoestrogen genistein and hormone replacement therapy on homocysteine and C-reactive protein level in postmenopausal women. Acta Obstet Gynecol Scand. 2005; 84:474–477. PMID: 15842212.
67. Yildiz MF, Kumru S, Godekmerdan A, Kutlu S. Effects of raloxifene, hormone therapy, and soy isoflavone on serum high-sensitive C-reactive protein in postmenopausal women. Int J Gynaecol Obstet. 2005; 90:128–133. PMID: 15970291.

68. Clerici C, Setchell KD, Battezzati PM, Pirro M, Giuliano V, Asciutti S, et al. Pasta naturally enriched with isoflavone aglycons from soy germ reduces serum lipids and improves markers of cardiovascular risk. J Nutr. 2007; 137:2270–2278. PMID: 17885010.

69. Aubertin-Leheudre M, Lord C, Khalil A, Dionne IJ. Effect of 6 months of exercise and isoflavone supplementation on clinical cardiovascular risk factors in obese postmenopausal women: a randomized, double-blind study. Menopause. 2007; 14:624–629. PMID: 17290158.
70. Salminen S, Bouley C, Boutron-Ruault MC, Cummings JH, Franck A, Gibson GR, et al. Functional food science and gastrointestinal physiology and function. Br J Nutr. 1998; 80(Suppl 1):S147–S171. PMID: 9849357.

71. Nova E, Wärnberg J, Gómez-Martínez S, Díaz LE, Romeo J, Marcos A. Immunomodulatory effects of probiotics in different stages of life. Br J Nutr. 2007; 98(Suppl 1):S90–S95. PMID: 17922968.

72. Olivares M, Díaz-Ropero MP, Gómez N, Lara-Villoslada F, Sierra S, Maldonado JA, et al. The consumption of two new probiotic strains, Lactobacillus gasseri CECT 5714 and Lactobacillus coryniformis CECT 5711, boosts the immune system of healthy humans. Int Microbiol. 2006; 9:47–52. PMID: 16636989.
73. Olivares M, Paz Díaz-Ropero M, Gómez N, Sierra S, Lara-Villoslada F, Martín R, et al. Dietary deprivation of fermented foods causes a fall in innate immune response. Lactic acid bacteria can counteract the immunological effect of this deprivation. J Dairy Res. 2006; 73:492–498. PMID: 16987435.

74. Kekkonen RA, Lummela N, Karjalainen H, Latvala S, Tynkkynen S, Jarvenpaa S, et al. Probiotic intervention has strain-specific anti-inflammatory effects in healthy adults. World J Gastroenterol. 2008; 14:2029–2036. PMID: 18395902.

75. Pineiro M, Asp NG, Reid G, Macfarlane S, Morelli L, Brunser O, et al. FAO technical meeting on prebiotics. J Clin Gastroenterol. 2008; 42(Suppl 3):(Pt 2):S156–S159. PMID: 18685504.

76. Guigoz Y, Rochat F, Perruisseau-Carrier G, Rochat I, Schiffrin EJ. Effects of oligosaccharide on the faecal flora and non-specific immune system in elderly people. Nutr Res. 2002; 22:13–25.

77. Schiffrin EJ, Thomas DR, Kumar VB, Brown C, Hager C, Van't Hof MA, et al. Systemic inflammatory markers in older persons: the effect of oral nutritional supplementation with prebiotics. J Nutr Health Aging. 2007; 11:475–479. PMID: 17985062.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download