Abstract
Purpose
The aim of this study was to compare serum leptin, neuropeptide Y (NPY), and islet amyloid polypeptide (amylin) levels in obese and normal weight children, and to investigate their correlations with anthropometric parameters and metabolic bio-marker levels.
Methods
Body mass index (BMI), waist and hip circumference, blood pressure (systolic/diastolic), lipid profile, fasting glucose, and serum insulin, leptin, NPY, and amylin levels were measured in 56 children (24 obese children and 32 non-obese controls). Homeostatic model assessment-insulin resistance (HOMA-IR) values were calculated and the relationships between anthropometric variables, metabolic biomarkers, and diet-regulating factors (leptin, NPY, and amylin levels) were examined.
Results
BMI, hip circumference, waist circumference, and systolic and diastolic pressure were significantly higher in the obese group than in the non-obese group (p<0.0001). Total cholesterol, triglyceride, low-density lipoprotein-cholesterol, glucose, and insulin levels were also significantly higher in the obese group (p<0.05). On the other hand, high-density lipoprotein-cholesterol levels were higher in the non-obese group , but this was not significant. Serum leptin, NPY, and amylin levels were significantly higher in the obese group (p<0.05). Furthermore, in the obese group, leptin levels were found to be significantly correlated with BMI (r=0.379, p=0.043), and NPY levels (r=0.377, p=0.044), and amylin levels were found to be significantly correlated with insulin levels (r=0.400, p=0.048), and HOMA-IR (r=0.459, p=0.028).
The prevalence of obesity is increasing worldwide, and its rapidly increasing prevalence among children and adolescents is of considerable social concern [1]. In the majority, childhood obesity leads to adult obesity, and furthermore, obese children develop early complications of metabolic disorder, such as, hyperinsulinemia, hyperlipidemia, and hypertension [2]. These facts and findings indicate that the management of obesity by prevention and proper treatment should be started in childhood. Diverse methods, such as, medication, dietary treatment, exercise, and eating habit changes have been used to treat obesity. Furthermore, various central and peripheral regulators of appetite have been found to play a role in food intake regulation. Many studies have been conducted with a view toward using such factors as anti-obesity agents. Leptin, one of these diet-regulating factors, is synthesized and secreted by adipocytes, and suppresses appetite and promotes energy consumption via the hypothalamus [3,4]. Serum leptin levels have been reported to be positively correlation with body fat percentages [5], and leptin levels in obese children were found to be increased [6]. Neuropeptide Y (NPY) is found in the hypothalamus and is a potent orexigenic signal among various neurotransmitters [7]. Based on this knowledge, a NPY receptor antagonist was developed to inhibit food intake. However, the inhibition of food intake is now known to have less effect on appetite suppression than was originally expected [8]. Amylin is produced by beta cells of pancreas, and suppresses appetite and glucagon secretion. Furthermore, it has been reported that obese people have higher amylin serum levels than normal people [9]. Many studies have been conducted on leptin resistance among obese children, whereas relatively few studies have been conducted on NPY and amylin, which are known to participate in dietary regulation. Against this background, we undertook to examine the natures of relations between diet regulating factors and changes in body composition, biochemical factors (blood glucose, insulin and lipid level), and diet-regulating factors (leptin, NPY, and amylin) in obese children.
Twenty-four obese children (13 boys and 11 girls) aged between 8 and 12 years with a 95 percentile body mass index (BMI; kg/m2) by sex and age were recruited from pre-pubertal children who visited the children's obesity clinic at Chosun University Hospital in the period June to August in 2009. The exclusion criteria applied were; symptomatic obesity, such as, diabetes, Prader-Willi syndrome, polycystic ovarian syndrome, Cushing syndrome, and hypothyroidism. The control group included 32 age-matched children (18 boys and 14 girls) of normal weight with no relevant medical history.
Height was measured to 0.1 cm using a Harpenden stadiometer, weight was measured to 0.1 kg without outer clothing, and BMI was calculated by dividing weight (kg) by height squared (m2). Calculated BMIs were compared with BMIs by sex and age published by the Korean Pediatric Society in 2007. When a BMI was at the 95 percentile or higher, the subject was assigned to the obese group, and when less, the subject was assigned to the normal weight group. Waist circumference was measured at the highest point of both iliac crests during exhalation, and hip circumference was defined as the maximum horizontal measure with the subject standing straight. A mercury sphygmomanometer was used to measure systolic and diastolic blood pressures.
Blood was collected after fasting for 12 hours in order to measure blood glucose and insulin levels. As an indicator of insulin resistance, homeostatic model assessment-insulin resistances [(HOMA-IR; glucose (mmol/L)xinsulin (µIU/mL)/22.5] were calculated. An auto-analyzer (ADVIA 2400; JEOL Ltd., Tokyo, Japan) was used to measure total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and triglyceride levels. Blood samples were stored in a freezer (at -20℃) before performing the analyses below. Serum leptin levels were determined using a human leptin Q ELISA kit (R & D Systems Inc., Minneapolis, MN, USA), NPY levels using a human neuropeptide Y ELISA kit (Linco Research, St. Charles, MO, USA), and amylin levels using a human amylin total ELISA kit (RayBiotech Inc., Norcross, GA, USA).
The student's t-test was used to compare group anthropometric data and blood test results. Pearson's correlation coefficients were used to compare diet-regulating factors (leptin, NPY and amylin), anthropometric data, and biochemical test indicators. Statistical significance was accepted for p-values <0.05.
Mean weights of obese and normal children were 52.4±0.39 kg and 29.9±0.20 kg, respectively (p<0.0001), and means heights were 143.9±0.37 cm and 133.7±0.36 cm, respectively (p<0.0001). Thus, mean BMIs were 25.1±0.11 kg/m2 and 16.5±0.05 kg/m2, respectively (p<0.0001). Mean waist and hip circumferences were 91.0±0.31 cm and 83.1±0.31 cm, respectively, for obese children and 67.6±0.21 cm and 57.4±0.13 cm, respectively, for normal children, both of which were significantly higher for obese children (p<0.0001, Table 1). Mean systolic and diastolic blood pressures were 108.7±0.31 mmHg and 70.8±0.30 mmHg, respectively, for obese children and 104.0±0.25 mmHg and 65.7±0.23 mmHg, respectively, for normal weight children (both p<0.02, Table 1). All data is presented as mean±standard deviation.
Mean serum total cholesterol, triglyceride, and LDL cholesterol levels were 167.9±1.13 mg/dL, 163.0±3.83 mg/dL, and 84.3±1.16 mg/dL, respectively, for obese children and 141.0±0.94 mg/dL, 62.9±1.11 mg/dL, and 64.9±0.70 mg/dL, respectively, for normal children (all p<0.05). Mean fasting blood glucose, insulin level, and HOMA-IR were 95.4±0.60 mg/dL, 13.5±0.54 µIU/mL, and 3.27±0.16, respectively, for obese children and 73.6±0.66 mg/dL, 5.76±0.14 µIU/mL, and 0.78±0.02, respectively, for normal children (all p<0.05, Table 2). Mean HDL cholesterol was 35.5±0.33 mg/dL for obese children and 40.1±0.42 mg/dL for normal children, but this difference was not significant (p=0.07, Table 2).
For obese children, serum levels of leptin, NPY, and amylin were 9.21±0.24 pg/mL, 301.0±2.59 pM, and 4.41±0.11 pM, respectively, and for normal children, these were 0.85±0.02 pg/mL, 276.2±1.14 pM, and 3.09±0.05 pM, respectively. Therefore, obese children had significantly higher levels than normal children (p<0.05, Fig. 1A-C).
Regarding, correlations with other factors, such as, leptin, NPY, and amylin levels, anthropometric data, and biochemical test indicators in normal children, leptin levels were found to be significantly correlated with NPY levels (r=-0.959, p=0.000), amylin levels (r=-0.784, p=0.000), and LDL cholesterol levels (r=0.439, p=0.014). Furthermore, NPY levels were significantly correlated with amylin levels (r=0.784, p=0.000). Among obese children, leptin levels were significantly correlated with BMIs (r=0.379, p=0.043) and NPY levels (r=0.377, p=0.044) (Fig. 2), but not with amylin levels (r=0.230, p=0.126). In obese children, NPY levels were not correlated with amylin levels (r =0.290, p=0.063). However, amylin levels were significantly correlated with insulin levels (r=0.400, p=0.048) and HOMA-IR values (r=0.459, p=0.028) (Fig. 3).
Recently, the nutritional status of children and adolescents in South Korea has improved thanks to economic growth and dietary improvements. However, obesity among children and adolescents continues to increase because of eating habits and lack of exercise. The prevalences of overweightedness and obesity in Korea among children and adolescents aged 10-19 in 1998, 2001, and 2005 were found by Korean National Health and Nutrition Examination Surveys to be 16.4%, 13.8%, and 24.2%. Furthermore, obesity among middle and high school students increased from 8.6% in 2005 to 9.8% in 2007, according to the Korean Youth Health Risk Behavior On-line Survey. Obesity among children and adolescents is a global phenomenon and one of the most serious health problems faced by modern society, for example, in the US, UK, Italy, Japan, and China, the prevalence of obesity has more than tripled over 20-30 years [10]. Furthermore, pediatric obesity is closely related to the early development of associated complications (hyperinsulinemia, insulin resistance, and lipid metabolic disorder), type 2 diabetes, fatty liver, hypertension, and cardiovascular disease, and is a risk factor of adult obesity. The present study also shows that obese children have significantly higher systolic and diastolic blood pressures, and significantly higher fasting blood glucose and insulin, HOMA-IR, cholesterol, and LDL levels. Therefore, it appears likely that obesity had already caused the development of cardiovascular disorders and glucose and lipid metabolic disorders in these children.
Imbalance in appetite regulating factor is a cause of obesity associated with the occurrence of metabolic complications. The human body maintains weight and energy metabolism by using various hormones and neuromodulators, that is, central and peripheral regulators of appetite, to achieving a balance between appetite, energy intake, and consumption. The appetite regulating factors include hormones (e.g., leptin and adiponectin secreted by adipocytes), pancreatic hormone, intestinal hormones (e.g., ghrelin and cholecystokinin [CCK]) , and various regulators of appetite that act in the hypothalamus or brain stem. Moreover, if these factors are perturbed, homeostasis is lost, which is known to cause obesity and diverse relevant complications [11].
Leptin is an important hormone that participates in fat metabolism. Leptin reduces appetite and increases calorie consumption via feedback mechanisms to the appetite and satiety centers in hypothalamus, which regulate body weight [3,4]. However, if the leptin gene is mutated or leptin receptor is malfunctioning, appetite increases [6,12]. Furthermore, since serum leptin level is proportional to body fat mass, its level is markedly elevated in obese people [13]. In fact, many studies have reported that serum leptin levels are positively correlated with BMI, body fat mass, body fat percentage, and obesity [12,14]. Park et al. [15] found that leptin levels in blood were significantly correlated with subcutaneous fat and visceral fat levels, fasting blood glucose and insulin levels, and insulin resistance, and that they were more strongly correlated with subcutaneous fat than visceral fat.
The results of this study also show that serum leptin levels are significantly higher in obese than in children of normal weight. Furthermore, leptin levels in obese children were found to be significantly correlated with BMIs and NPY levels. On the other hand, leptin levels were not found to be correlated with lipid levels or insulin resistance.
NPY is a neurotransmitter found in the brain that increases appetite and lowers metabolic rate, which eventually leads to obesity [16,17]. NPY also prevents heat production from brown fat and suppresses the stimulation of sympathetic nerves and the hypothalamic-pituitary-thyroid axis to reduce energy consumption [18,19]. In addition, regardless of food intake, NPY stimulates the secretion of basal glucose insulin and blood cortisol in the morning [20]. One animal study demonstrated the effect of insulin resistance suppression by injecting a NPY antagonist into the brain [21]. Furthermore, previous studies that targeted normal children, leptin (an appetite-inhibiting factor) was found to suppress the generation of NPY (an appetite-stimulating factor) [22,23]. In the present study, serum NPY levels were significantly higher in obese children. An increase in leptin and the lack of NPY suppression in obese children suggest an imbalance between leptin and NPY, which could be considered an appetite-regulating center disorder. We suggest that additional studies be conducted to determine whether imbalances diet regulating factors can be used to predict the early onset of the complications of pediatric obesity.
Amylin is a pancreatic hormone that is secreted with insulin by beta cells, and can compensate the role of insulin via various mechanisms. Representative mechanisms include regulation of food intake, suppression of gastric acid secretion, and inhibition of glucagon release after a meal, and delayed gastric emptying [24]. In particular, amylin is used to treat type 2 diabetes due to its effects on gastric emptying and to its inhibition of glucagon secretion [25-27]. Amylin also regulates energy homeostasis and increases energy consumption, which has attracted attention with respect to its possible use as an anti-obesity drug. Amylin regulates energy homeostasis in two ways, that is, by sending satiety or a fat signal. Several studies have shown that amylin sends a strong satiety signal to regulate food intake [24,27,28] and others have shown that an amylin injection reduces meal bolus within a few minutes [28] and that an amylin receptor antagonist injection increases meal bolus [29,30]. The effect of amylin on food intake regulation differs from that of CCK, which is a representative satiety hormone. The reduction in food intake elicited by a CCK is not observed when it is injected continuously, and thus, its effect on diet regulation is limited [31]. Furthermore, a single CCK injection reduced meal bolus but increased meal numbers, and thus, total food intake remained unchanged. However, when amylin was injected continuously, no compensatory increase in the number of meals was observed despite a reduction in food intake, and thus, amylin is considered a more effective diet-regulating factor [32,33]. In addition to its effects on satiety, amylin also reacts with body fat, like leptin and insulin [34,35] and it has been reported that basal blood amylin levels are higher in obese children [36] and thus, suggested that amylin could reduce weight increases by reducing body fat [37,38]. In a study by Lutz [27], body fat increased but body weight was changed when amylin antagonist was injected chronically. The present study also shows that amylin levels were significantly higher in obese children and that amylin levels in obese children were significantly correlated with insulin resistance. Furthermore, in one study of the use of amylin to treat adult obesity and type II diabetes, it was found that amylin treatment reduced body fat and induced weight loss [39,40]. However, amylin treatment can cause side effects, such as, nausea, vomiting, loss of appetite or hypoglycemia, which are of concern when amylin is considered to treat overweightedness in children [41]. Further studies are required to examine when amylin is administered for a protracted period to children.
This study is limited by the small number of patients included, which precluded comparisons between the pre- and post-pubertal periods and analyses to determine the effects of sex and body fat mass.
Nonetheless, this study confirms that obese children develop cardiovascular, glucose metabolism, and lipid metabolism disorders and that they develop leptin, NPY, and amylin resistance. Furthermore, the study confirms that serum amylin levels and insulin resistance are correlated.
We suggest that: 1) A prospective study be undertaken to investigate complications associated with obesity, changes in various diet-regulating factors (including other intestinal hormones, such as, ghrelin and pancreatic peptide) in a larger cohort of obese children. 2) A study be undertaken to examine changes in diet factors and metabolic indicators when obesity is treated by diet and exercise. 3) That the anti-obesity effects of leptin, NPY antagonist, and/or amylin be investigated in obese children.
Figures and Tables
Fig. 1
Comparisons of diet-regulating factors in the normal control and obese groups. Values are presented as mean±standard deviation. (A) Leptin, (B) Neuropeptide Y, (C) Amylin.
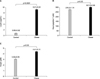
Fig. 2
The correlation between leptin levels and BMIs (A) and NPY levels (B) in the obese group. BMI: body mass index, NPY: neuropeptide Y.

Fig. 3
Correlations between amylin and insulin levels (A), and between amylin levels and HOMA-IR values (B) in the obese group. HOMA-IR: homeostatic model assessment-insulin resistance.

References
1. Strauss RS, Pollack HA. Epidemic increase in childhood overweight, 1986-1998. JAMA. 2001. 286:2845–2848.

2. Sinaiko AR, Donahue RP, Jacobs DR Jr, Prineas RJ. The Minneapolis Children's Blood Pressure Study. Relation of weight and rate of increase in weight during childhood and adolescence to body size, blood pressure, fasting insulin, and lipids in young adults. Circulation. 1999. 99:1471–1476.

3. Blum WF, Englaro P, Hanitsch S, Juul A, Hertel NT, Müller J, et al. Plasma leptin levels in healthy children and adolescents: dependence on body mass index, body fat mass, gender, pubertal stage, and testosterone. J Clin Endocrinol Metab. 1997. 82:2904–2910.

4. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994. 372:425–432.

5. Saranac L, Bjelakovic B, Stamenkovic H, Kamenov B. Orexitropic signaling proteins in obese children. ScientificWorldJournal. 2007. 7:1263–1271.

6. Clément K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998. 392:398–401.

7. Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000. 404:661–671.

8. Yang K, Guan H, Arany E, Hill DJ, Cao X. Neuropeptide Y is produced in visceral adipose tissue and promotes proliferation of adipocyte precursor cells via the Y1 receptor. FASEB J. 2008. 22:2452–2464.

9. Reinehr T, Kiess W, Kapellen T, Andler W. Insulin sensitivity among obese children and adolescents, according to degree of weight loss. Pediatrics. 2004. 114:1569–1573.

10. Baek S. Do obese children exhibit distinguishable behaviours from normal weight children?-based on literature review. Korean J Community Nutr. 2008. 13:386–395.
11. Stanley S, Wynne K, McGowan B, Bloom S. Hormonal regulation of food intake. Physiol Rev. 2005. 85:1131–1158.

12. Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996. 334:292–295.

13. Campfield LA, Smith FJ, Burn P. The OB protein (leptin) pathway--a link between adipose tissue mass and central neural networks. Horm Metab Res. 1996. 28:619–632.

14. Roemmich JN, Rogol AD. Role of leptin during childhood growth and development. Endocrinol Metab Clin North Am. 1999. 28:749–764.

15. Park KG, Park KS, Kim MJ, Kim HS, Suh YS, Ahn JD, et al. Relationship between serum adiponectin and leptin concentrations and body fat distribution. Diabetes Res Clin Pract. 2004. 63:135–142.

16. Allen YS, Adrian TE, Allen JM, Tatemoto K, Crow TJ, Bloom SR, et al. Neuropeptide Y distribution in the rat brain. Science. 1983. 221:877–879.

17. Stanley BG, Kyrkouli SE, Lampert S, Leibowitz SF. Neuropeptide Y chronically injected into the hypothalamus: a powerful neurochemical inducer of hyperphagia and obesity. Peptides. 1986. 7:1189–1192.

18. Billington CJ, Briggs JE, Grace M, Levine AS. Effects of intracerebroventricular injection of neuropeptide Y on energy metabolism. Am J Physiol. 1991. 260:R321–R327.

19. Fekete C, Sarkar S, Rand WM, Harney JW, Emerson CH, Bianco AC, et al. Agouti-related protein (AGRP) has a central inhibitory action on the hypothalamic-pituitary-thyroid (HPT) axis; comparisons between the effect of AGRP and neuropeptide Y on energy homeostasis and the HPT axis. Endocrinology. 2002. 143:3846–3853.

20. Moltz JH, McDonald JK. Neuropeptide Y: direct and indirect action on insulin secretion in the rat. Peptides. 1985. 6:1155–1159.

21. Yi CX, Foppen E, Abplanalp W, Gao Y, Alkemade A, la Fleur SE, et al. Glucocorticoid signaling in the arcuate nucleus modulates hepatic insulin sensitivity. Diabetes. 2012. 61:339–345.

22. Rohner-Jeanrenaud F, Cusin I, Sainsbury A, Zakrzewska KE, Jeanrenaud B. The loop system between neuropeptide Y and leptin in normal and obese rodents. Horm Metab Res. 1996. 28:642–648.

23. Smith FJ, Campfield LA, Moschera JA, Bailon PS, Burn P. Feeding inhibition by neuropeptide Y. Nature. 1996. 381:415–418.

24. Young A, Denaro M. Roles of amylin in diabetes and in regulation of nutrient load. Nutrition. 1998. 14:524–527.
25. Chapman I, Parker B, Doran S, Feinle-Bisset C, Wishart J, Strobel S, et al. Effect of pramlintide on satiety and food intake in obese subjects and subjects with type 2 diabetes. Diabetologia. 2005. 48:838–848.

26. Hollander P, Maggs DG, Ruggles JA, Fineman M, Shen L, Kolterman OG, et al. Effect of pramlintide on weight in overweight and obese insulin-treated type 2 diabetes patients. Obes Res. 2004. 12:661–668.

29. Schwartz MW, Woods SC, Seeley RJ, Barsh GS, Baskin DG, Leibel RL. Is the energy homeostasis system inherently biased toward weight gain? Diabetes. 2003. 52:232–238.

30. Woods SC. Gastrointestinal satiety signals I. An overview of gastrointestinal signals that influence food intake. Am J Physiol Gastrointest Liver Physiol. 2004. 286:G7–G13.

31. Lutz TA, Geary N, Szabady MM, Del Prete E, Scharrer E. Amylin decreases meal size in rats. Physiol Behav. 1995. 58:1197–1202.

32. Mollet A, Gilg S, Riediger T, Lutz TA. Infusion of the amylin antagonist AC 187 into the area postrema increases food intake in rats. Physiol Behav. 2004. 81:149–155.

33. Reidelberger RD, Haver AC, Arnelo U, Smith DD, Schaffert CS, Permert J. Amylin receptor blockade stimulates food intake in rats. Am J Physiol Regul Integr Comp Physiol. 2004. 287:R568–R574.

35. Roth JD, Hughes H, Kendall E, Baron AD, Anderson CM. Antiobesity effects of the beta-cell hormone amylin in diet-induced obese rats: effects on food intake, body weight, composition, energy expenditure, and gene expression. Endocrinology. 2006. 147:5855–5864.

36. Crawley JN, Beinfeld MC. Rapid development of tolerance to the behavioural actions of cholecystokinin. Nature. 1983. 302:703–706.

37. Arnelo U, Permert J, Adrian TE, Larsson J, Westermark P, Reidelberger RD. Chronic infusion of islet amyloid polypeptide causes anorexia in rats. Am J Physiol. 1996. 271:R1654–R1659.

38. Lutz TA, Mollet A, Rushing PA, Riediger T, Scharrer E. The anorectic effect of a chronic peripheral infusion of amylin is abolished in area postrema/nucleus of the solitary tract (AP/NTS) lesioned rats. Int J Obes Relat Metab Disord. 2001. 25:1005–1011.

39. Ravussin E, Smith SR, Mitchell JA, Shringarpure R, Shan K, Maier H, et al. Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity (Silver Spring). 2009. 17:1736–1743.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download